J Breast Cancer.
2014 Mar;17(1):61-68.
Efficacy and Safety Profile of Combining Sorafenib with Chemotherapy in Patients with HER2-Negative Advanced Breast Cancer: A Meta-analysis
- Affiliations
-
- 1Department of Breast and Thyroid Surgery, West China Hospital, Sichuan University, Chengdu, China.
- 2Department of Breast Disease, The Second People's Hospital of Sichuan, Chengdu, China.
- 3Department of General Surgery, West China Hospital, Sichuan University, Chengdu, China. chengnsdoctor@126.com
- 4Tumor Molecular Laboratory, West China Hospital, Sichuan University, Chengdu, China.
- 5Department of Radiology, West China Hospital, Sichuan University, Chengdu, China.
Abstract
- PURPOSE
The aim of the study was to evaluate the efficacy and safety of combining sorafenib with chemotherapy in patients with human epidermal growth factor receptor 2 (HER2)-negative advanced breast cancer.
METHODS
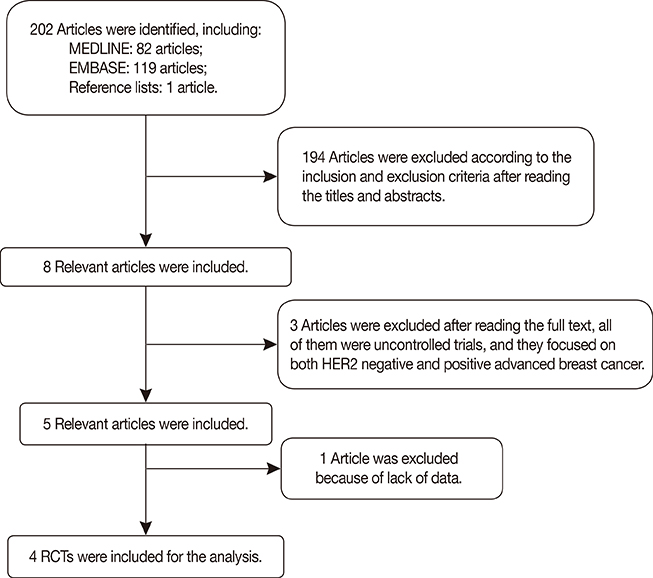
MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, American Society for Clinical Oncology abstracts, and European Society for Medical Oncology abstracts were searched. Randomized clinical trials that compared the efficacy and safety of sorafenib plus chemotherapy in patients with HER2-negative advanced breast cancer with placebo plus chemotherapy were eligible. The endpoints were progression-free survival (PFS), overall survival (OS), time to progression (TTP), duration of response (DOR), overall response rate (ORR), clinical benefits, and adverse effects. The meta-analysis was performed using Review Manager 5.2.6 (The Nordic Cochrane Centre), and the fixed-effect model weighted by the Mantel-Haenszel method was used. When considerable heterogeneity was found (p<0.1), further analysis (subgroup analysis, sensitivity analysis, or random-effect model) was performed to identify the potential cause. The results are expressed as hazard ratios or risk ratios, with their corresponding 95% confidence intervals.
RESULTS
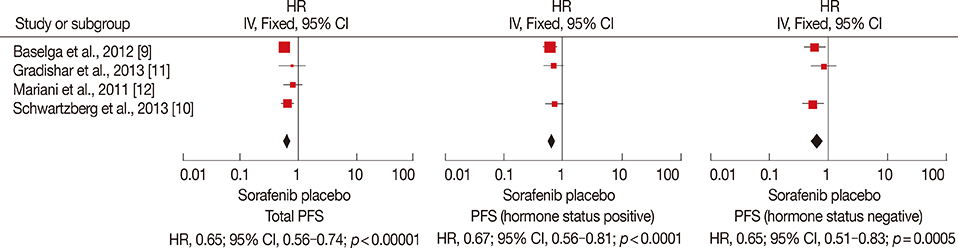
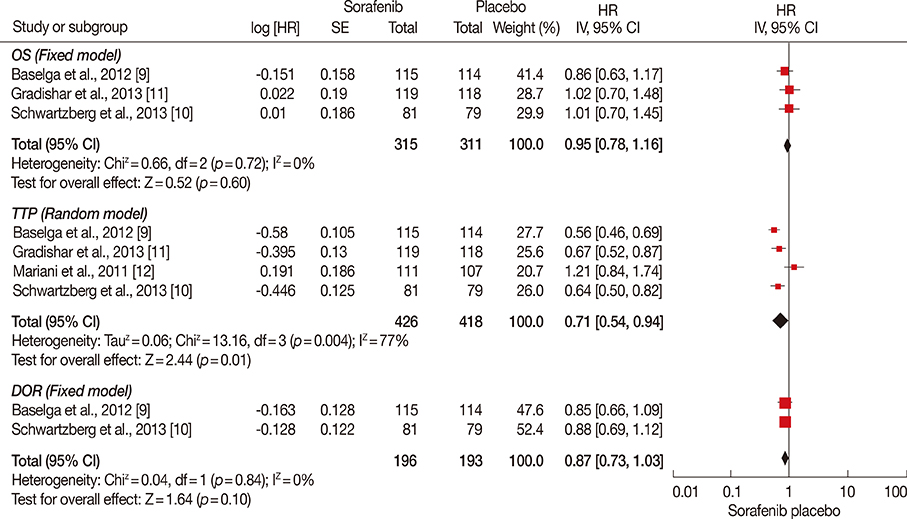
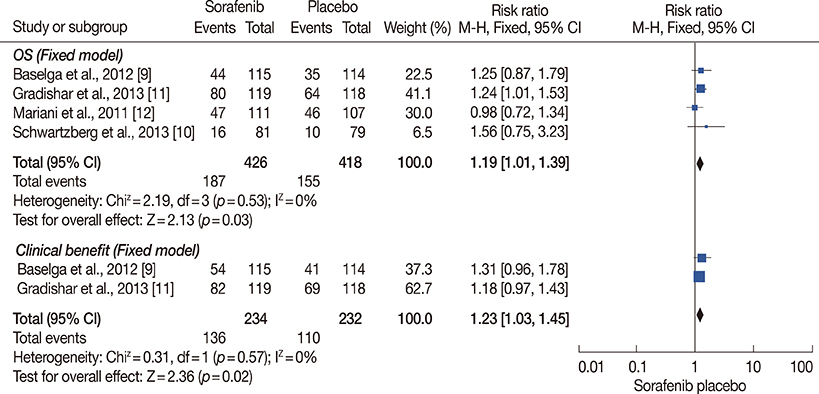
The final analysis included four trials comprising 844 patients. The results revealed longer PFS and TTP, and higher ORR and clinical benefit rates in patients receiving sorafenib combined with chemotherapy compared to those receiving chemotherapy and placebo. OS and DOR were similar in the two groups. Meanwhile, the incidence of some adverse effects, including hand-foot skin reaction/hand-foot syndrome, diarrhea, rash, and hypertension, were significantly higher in the sorafenib arm.
CONCLUSION
Sorafenib combined with chemotherapy may prolong PFS and TTP. This treatment was associated with manageable toxicities, but frequent dose interruptions and reductions were required.
Keyword
MeSH Terms
Figure
Reference
-
1. Evans WP. Breast cancer screening: successes and challenges. CA Cancer J Clin. 2012; 62:5–9.
Article2. Gao S, Barber B, Schabert V, Ferrufino C. Tumor hormone/HER2 receptor status and pharmacologic treatment of metastatic breast cancer in Western Europe. Curr Med Res Opin. 2012; 28:1111–1118.
Article3. Gradishar WJ. Sorafenib in locally advanced or metastatic breast cancer. Expert Opin Investig Drugs. 2012; 21:1177–1191.
Article4. Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013; 274:113–126.
Article5. Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005; 23:1782–1790.
Article6. Reddy S, Raffin M, Kaklamani V. Targeting angiogenesis in metastatic breast cancer. Oncologist. 2012; 17:1014–1026.
Article7. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article8. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009; 27:3312–3318.
Article9. Baselga J, Segalla JG, Roché H, Del Giglio A, Pinczowski H, Ciruelos EM, et al. Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012; 30:1484–1491.
Article10. Schwartzberg LS, Tauer KW, Hermann RC, Makari-Judson G, Isaacs C, Beck JT, et al. Sorafenib or placebo with either gemcitabine or capecitabine in patients with HER-2-negative advanced breast cancer that progressed during or after bevacizumab. Clin Cancer Res. 2013; 19:2745–2754.
Article11. Gradishar WJ, Kaklamani V, Sahoo TP, Lokanatha D, Raina V, Bondarde S, et al. A double-blind, randomised, placebo-controlled, phase 2b study evaluating sorafenib in combination with paclitaxel as a first-line therapy in patients with HER2-negative advanced breast cancer. Eur J Cancer. 2013; 49:312–322.
Article12. Mariani G, Burdaeva O, Roman L, Staroslawska E, Udovitsa D, Driol P, et al. A double-blind, randomized phase lib study evaluating the efficacy and safety of sorafenib (SOR) compared to placebo (PL) when administered in combination with docetaxel and/or letrozole in patients with metastatic breast cancer (MBC): FM-B07-01 Trial. Eur J Cancer. 2011; 47:Suppl 2. 10.
Article13. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.2. Chichester: John Wiley & Sons;2009.14. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007; 8:16.
Article15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.
Article16. Ding Q, Huo L, Yang JY, Xia W, Wei Y, Liao Y, et al. Down-regulation of myeloid cell leukemia-1 through inhibiting Erk/Pin 1 pathway by sorafenib facilitates chemosensitization in breast cancer. Cancer Res. 2008; 68:6109–6117.
Article17. Merz M, Komljenovic D, Zwick S, Semmler W, Bäuerle T. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. Eur J Cancer. 2011; 47:277–286.
Article18. Bonelli MA, Fumarola C, Alfieri RR, La Monica S, Cavazzoni A, Galetti M, et al. Synergistic activity of letrozole and sorafenib on breast cancer cells. Breast Cancer Res Treat. 2010; 124:79–88.
Article19. Mina LA, Yu M, Johnson C, Burkhardt C, Miller KD, Zon R. A phase II study of combined VEGF inhibitor (bevacizumab+sorafenib) in patients with metastatic breast cancer: Hoosier Oncology Group Study BRE06-109. Invest New Drugs. 2013; 31:1307–1310.
Article20. Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM Jr, Wiesenfeld M, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009; 27:11–15.
Article21. Bianchi G, Loibl S, Zamagni C, Salvagni S, Raab G, Siena S, et al. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs. 2009; 20:616–624.
Article22. Baselga J, Costa F, Gomez H, Hudis CA, Rapoport B, Roche H, et al. A phase 3 trial comparing capecitabine in combination with sorafenib or placebo for treatment of locally advanced or metastatic HER2-negative breast cancer (the RESILIENCE study): study protocol for a randomized controlled trial. Trials. 2013; 14:228.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Serum HER2 Levels on Survival and Its Correlation with Clinicopathological Parameters in Women with Breast Cancer
- Experience and Expectation for Molecular Target Therapy in Hepatocellular Carcinoma
- Prognostic Value of the Evolution of HER2-Low Expression after Neoadjuvant Chemotherapy
- Updated Bayesian Network MetaAnalysis of Adjuvant Targeted Treatment Regimens for Early Human Epidermal Growth Factor Receptor-2 Positive Breast Cancer
- Treatments Other than Sorafenib for Patients with Advanced Hepatocellular Carcinoma