Transl Clin Pharmacol.
2015 Dec;23(2):42-45. 10.12793/tcp.2015.23.2.42.
Clearance
- Affiliations
-
- 1Department of Pharmacology & Clinical Pharmacology, University of Auckland, Auckland, New Zealand. n.holford@auckland.ac.nz
- 2Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 3PIPET (Pharmacometrics Institute for Practical Education and Training), College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2351806
- DOI: http://doi.org/10.12793/tcp.2015.23.2.42
Abstract
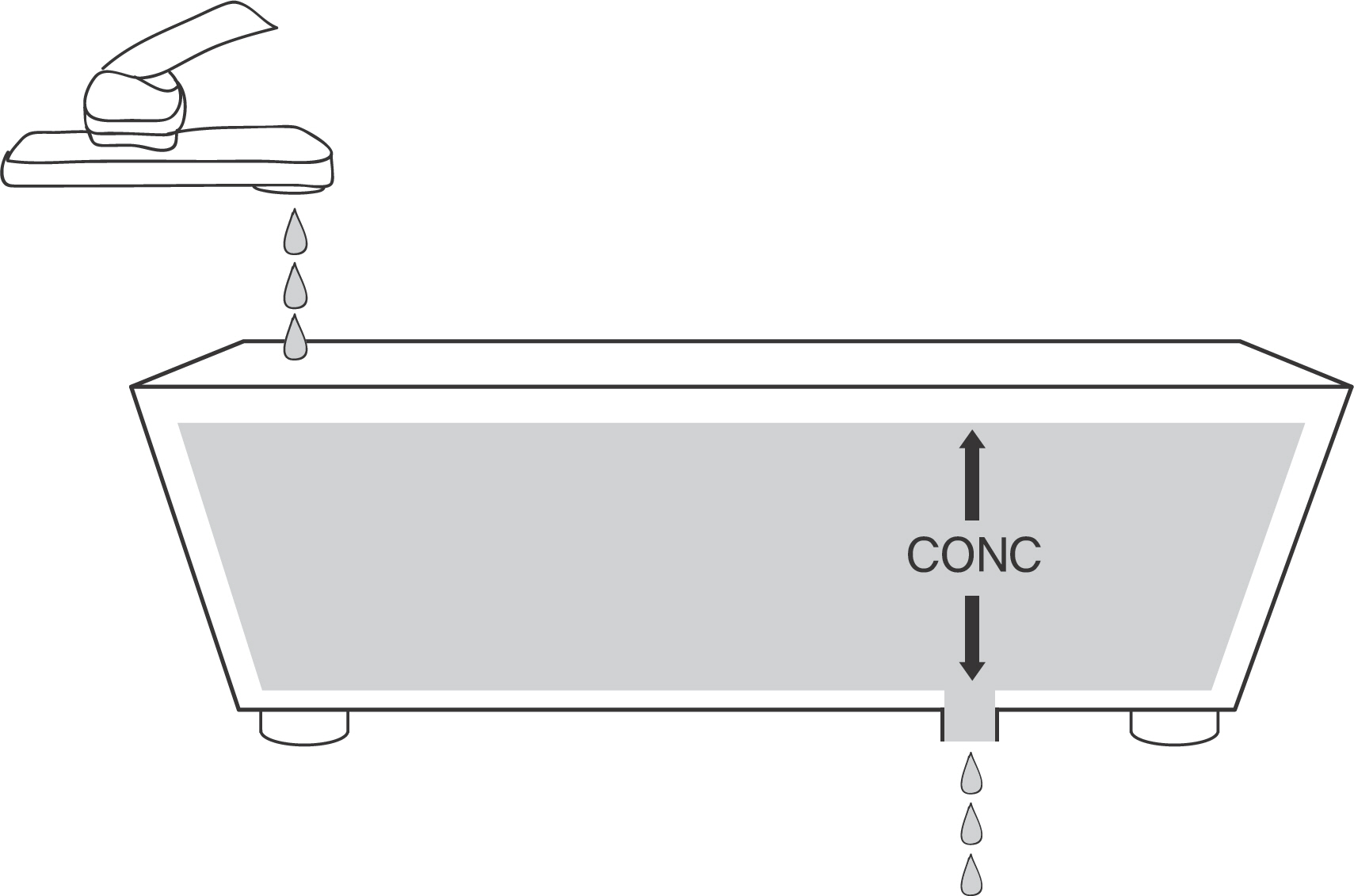
- This tutorial deals with basic concepts of clearance, the most important parameter in pharmacokinetics but often challenging for students in clinical pharmacology. Its relationships with dose, concentration and elimination rate are discussed using a physical model and examples of commonly used drugs, as well as its physiological aspects pertaining to the blood flow to differing organs. Finally, application of clearance to the calculation of maintenance dose rate and half-life is used to show how it is essential in pharmacotherapy and clinical pharmacology.
Keyword
Figure
Cited by 3 articles
-
Tutorial for beginners: the concept of clearance explained using the example of a vacuum cleaner
Dong-Seok Yim
Transl Clin Pharmacol. 2017;25(1):1-4. doi: 10.12793/tcp.2017.25.1.1.Pharmacodynamic principles and target concentration intervention
Nick Holford
Transl Clin Pharmacol. 2018;26(4):150-154. doi: 10.12793/tcp.2018.26.4.150.Treatment response and disease progression
Nick Holford
Transl Clin Pharmacol. 2019;27(4):123-126. doi: 10.12793/tcp.2019.27.4.123.
Reference
-
References
1. Holford N, Black P, Couch R, Kennedy J, Briant R. Theophylline target concentration in severe airways obstruction – 10 or 20 mg/L? A randomised concentration-controlled trial. Clin Pharmacokinet. 1993; 25:495–505.2. St⊘rset E, Holford N, Hennig S, Bergmann TK, Bergan S, Bremer S, et al. Improved prediction of tacrolimus concentrations early after kidney transplantation using theory-based pharmacokinetic modelling. Br J Clin Pharmacol. 2014; 78:509–523.
Article3. Lee MG, Chen ML, Huang SM, Chiou WL. Pharmacokinetics of drugs in blood I. Unusual distribution of gentamicin. Biopharm Drug Dispos. 1981; 2:89–97.
Article4. Marty J, Shaw J, Hunt D. The stability of glyceryl trinitrate tablets during patient use. Aust N Z J Med. 1983; 13:147–150.
Article5. Ferron LA, Weber JP, Hébert J, Bédard PM. Theophylline concentrations in serum, plasma, and whole blood compared. Clin Chem. 1985; 31:1415–1416.
Article6. Wikipedia. Order of reaction. 2015.7. Holford N, Jiang Y, Murry DJ, Brown TL, Gary Milavetz G. The Influence of Body Composition on Ethanol Pharmacokinetics using a Rate Dependent Extraction Model. PAGE. 2015; 24:3405. [. www.page-meeting.org/?abstract=3405].8. Cutler RE, Forland SC, Hammond PG, Evans JR. Extracorporeal removal of drugs and poisons by hemodialysis and hemoperfusion. Annu Rev Pharmacol Toxicol. 1987; 27:169–191.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Esophageal Motility and Acid Clearance in Patients with Esophageal Varices
- Effect of Hypertonic Seawater (Sinomarin(R)) on Mucociliary Clearance in Normal Subjects
- Change in the Nasal Patency and Mucociliary Clearance after Phenylephrine Spray
- Association of lactate clearance with outcomes of patients with gastrointestinal bleeding visiting the emergency department
- Studies on Renal function in Normal Korean: III. Endogenous Creatinine Chromogen Clearance on Normal Korean*