Transl Clin Pharmacol.
2016 Sep;24(3):115-118. 10.12793/tcp.2016.24.3.115.
Revisiting the well-stirred model of hepatic clearance: Q(H), CL(H) and F changing in the same direction
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, PIPET (Pharmacometrics Institute of Practical Education and Training), College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. yimds@catholic.ac.kr
- KMID: 2351795
- DOI: http://doi.org/10.12793/tcp.2016.24.3.115
Abstract
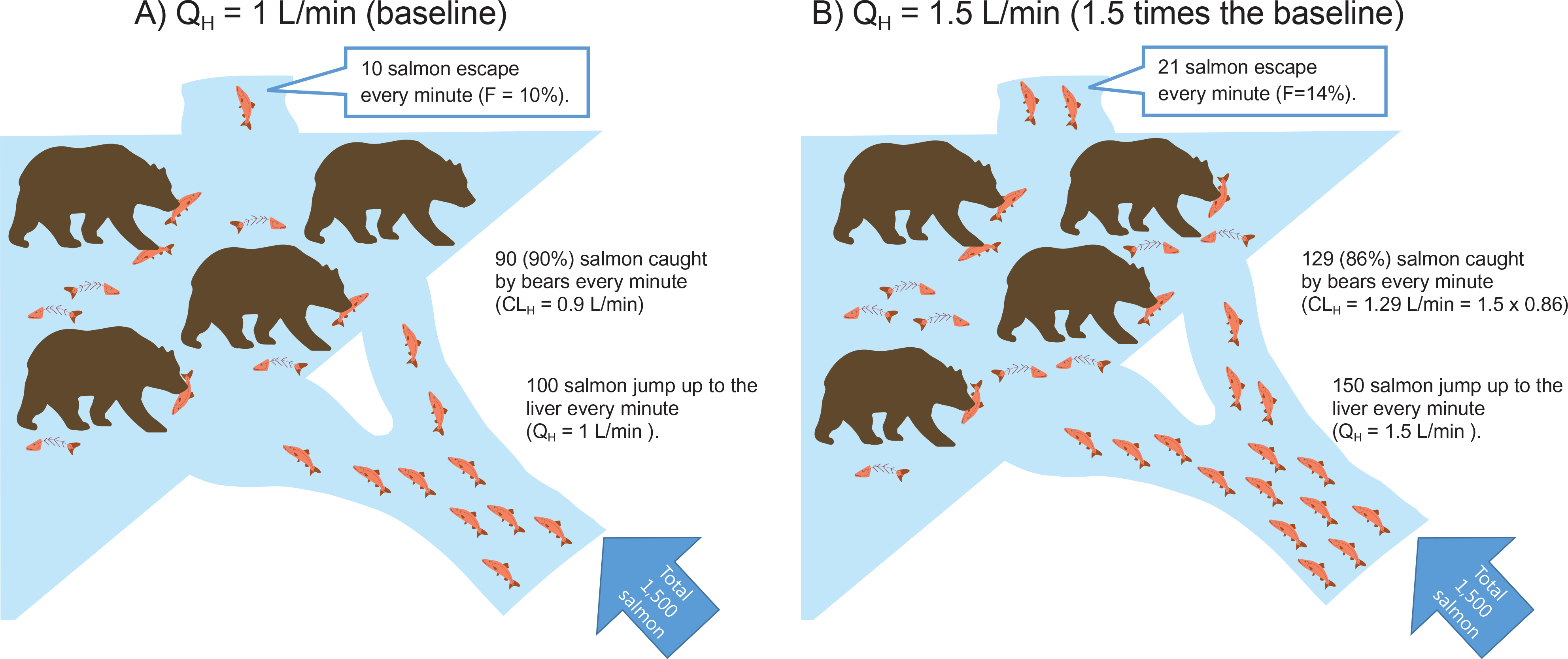
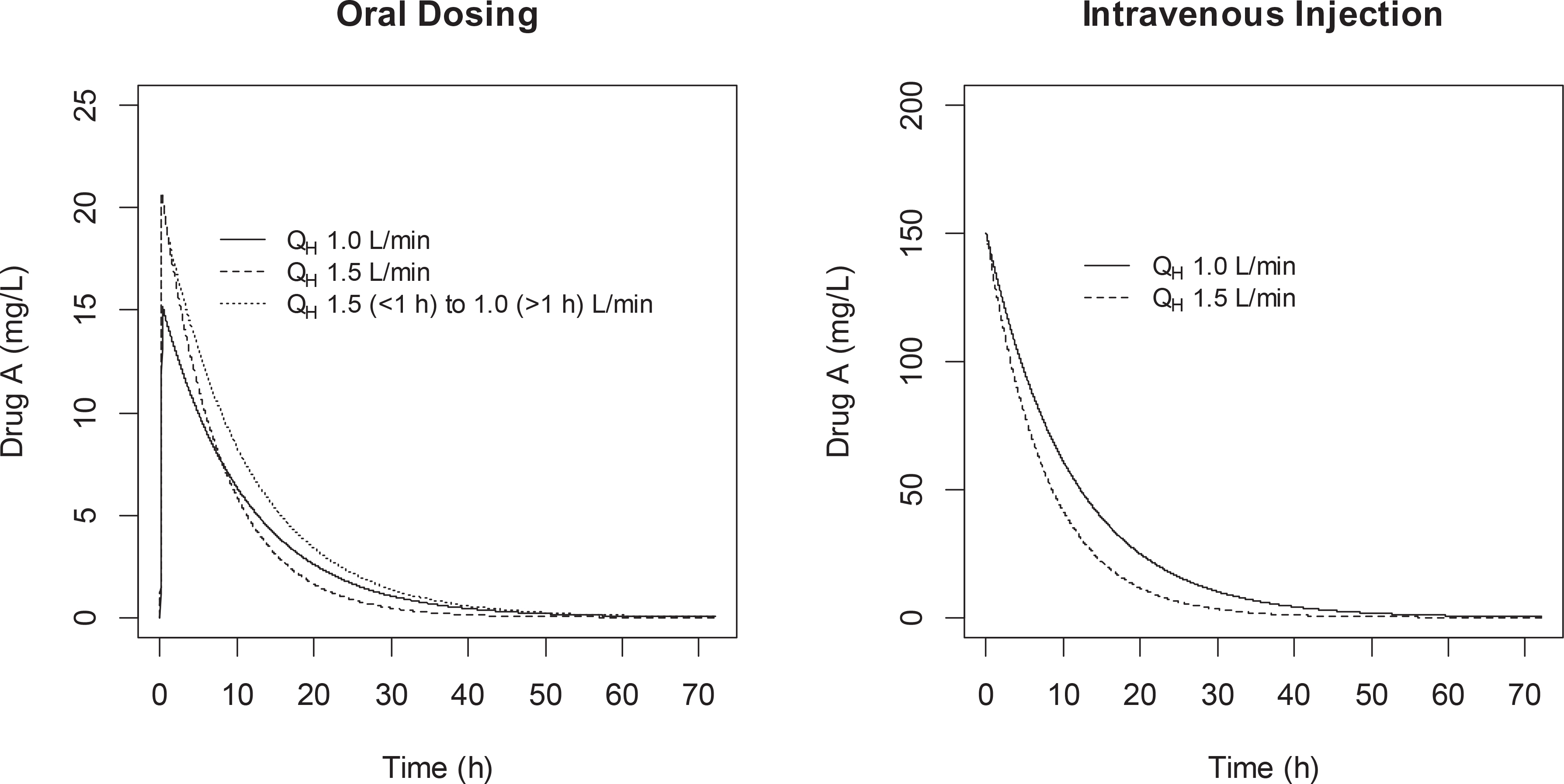
- This tutorial examines the relationship between CL, F, and hepatic blood flow (Q(H)) quantitatively at oral and i.v. administration as an answer to the quiz set for KSCPT members. In case of oral dosing, when hepatic blood flow increases, the hepatic clearance (CL) and bioavailability (F) increases in high-extraction ratio drugs according to the well-stirred model equations for hepatic clearance: CL(H) = Q(H)·ER = Q(H)·f(u)·CL(int)/(Q(H)+f(u)·CL(int)) and F = 1 - ER Despite such a clear relationship, many students may feel it rather paradoxical that the increased CL (thus decreasing the AUC) causes increased F and thus the AUC (F·Dose/CLH) remains unchanged. This tutorial clarifies that the degree to which CL increase fails to match that of the Q(H) increase, and thus the decreased ER (= CL/Q(H)) that results in the increased F. Contemplating this simple, but seemingly paradoxical phenomenon may help students gain a deeper understanding of the first-pass effect.
Figure
Reference
-
1.Rowland M., Tozer TN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. 4th ed.Lippincott Williams & Wilkins;Baltimore: 2011. p. 125–126.2.Rowland M., Tozer TN. Clinical pharmacokinetics and pharmacodynamics: concepts and applications. 4th ed.Lippincott Williams & Wilkins;Baltimore: 2011. 196.3.Olanoff LS., Walle T., Cowart TD., Walle UK., Oexmann MJ., Conradi EC. Food effects on propranolol systemic and oral clearance: support for a blood flow hypothesis. Clin Pharmacol Ther. 1986. 40:408–414.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Predicting human pharmacokinetics from preclinical data: clearance
- Estimation of GFR Using Iohexol Plasma Clearance in Korean without Renal Disease
- A Case of Congenital Vestibular Anomaly with Direction Changing Positional Nystagmus
- Loganin Ameliorates Acute Kidney Injury and Restores Tofacitinib Metabolism in Rats: Implications for Renal Protection and Drug Interaction
- A Comparative Study of how Subjects' Characteristics and Nursing Service Quality Influence on Hospital Revisiting Intent between Patients and Nurses