J Korean Med Sci.
2015 Nov;30(11):1567-1576. 10.3346/jkms.2015.30.11.1567.
Correlation between Drug Market Withdrawals and Socioeconomic, Health, and Welfare Indicators Worldwide
- Affiliations
-
- 1Seoul National University Biomedical Informatics (SNUBI) and Systems Biomedical Informatics Research Center, Division of Biomedical Informatics, Seoul National University College of Medicine, Seoul, Korea. juhan@snu.ac.kr
- KMID: 2351105
- DOI: http://doi.org/10.3346/jkms.2015.30.11.1567
Abstract
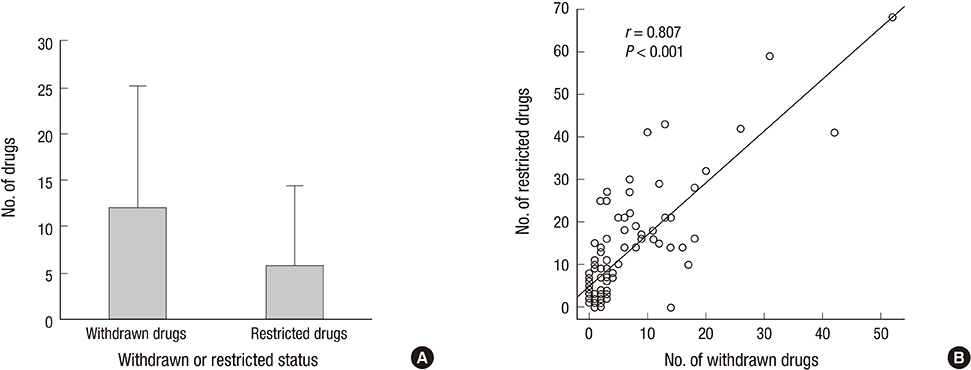
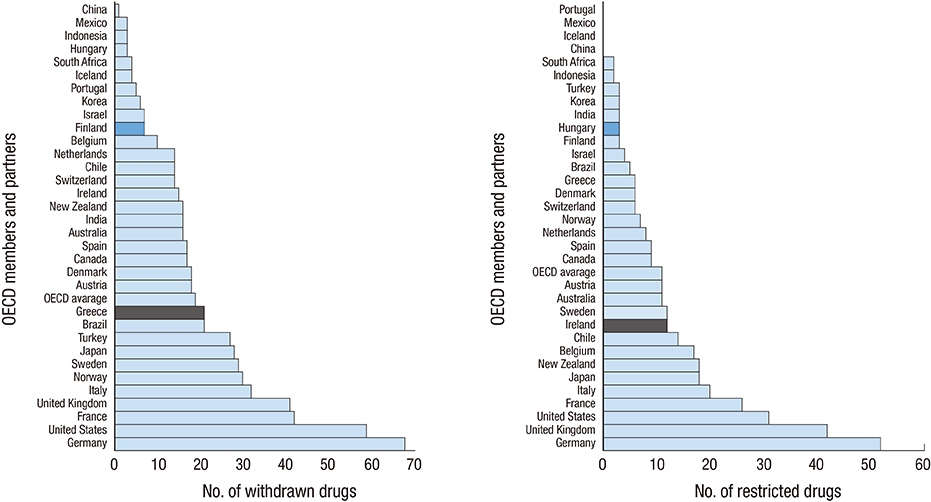
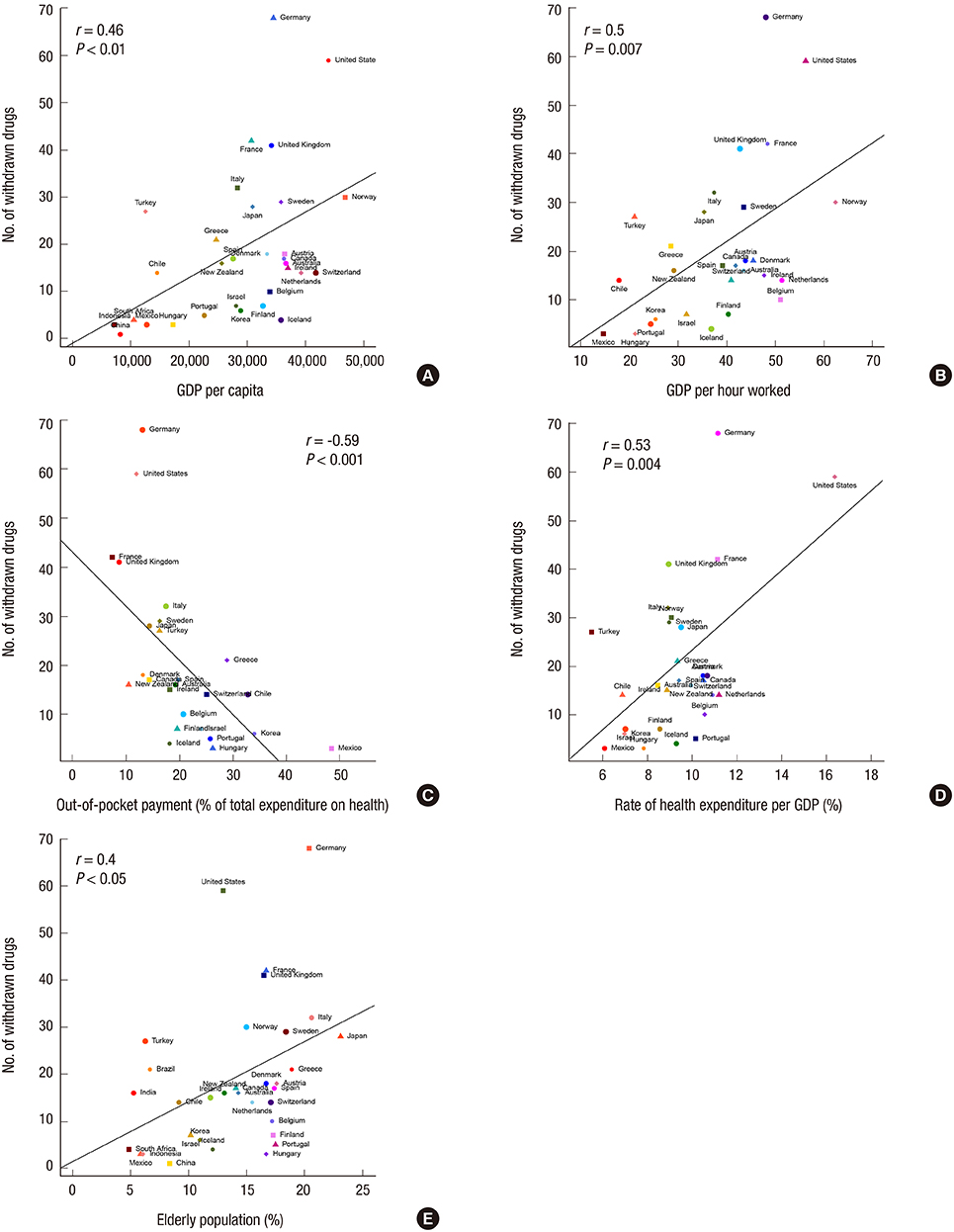
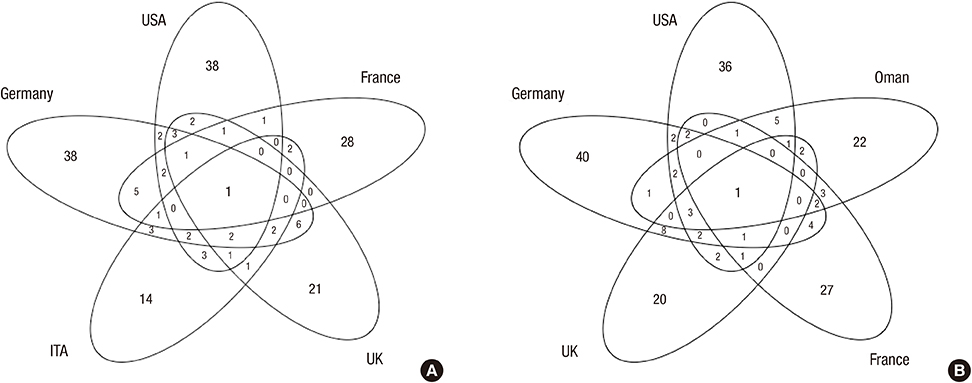
- The relationship between the number of withdrawn/restricted drugs and socioeconomic, health, and welfare indicators were investigated in a comprehensive review of drug regulation information in the United Nations (UN) countries. A total of of 362 drugs were withdrawn and 248 were restricted during 1950-2010, corresponding to rates of 12.02+/-13.07 and 5.77+/-8.69 (mean+/-SD), respectively, among 94 UN countries. A socioeconomic, health, and welfare analysis was performed for 33 OECD countries for which data were available regarding withdrawn/restricted drugs. The gross domestic product (GDP) per capita, GDP per hour worked, health expenditure per GDP, and elderly population rate were positively correlated with the numbers of withdrawn and restricted drugs (P<0.05), while the out-of-pocket health expenditure payment rate was negatively correlated. The number of restricted drugs was also correlated with the rate of drug-related deaths (P<0.05). The World Bank data cross-validated the findings of 33 OECD countries. The lists of withdrawn/restricted drugs showed markedly poor international agreement between them (Fleiss's kappa=-0.114). Twenty-seven drugs that had been withdrawn internationally by manufacturers are still available in some countries. The wide variation in the numbers of drug withdrawals and restrictions among countries indicates the need to improve drug surveillance systems and regulatory communication networks.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Child
Child, Preschool
Drug Utilization/*economics/statistics & numerical data
Female
Gross Domestic Product/*statistics & numerical data
*Health Status Indicators
Humans
Infant
Infant, Newborn
Internationality
*Life Expectancy
Male
Middle Aged
Product Surveillance, Postmarketing/*economics/statistics & numerical data
Safety-Based Drug Withdrawals/*economics/statistics & numerical data
Social Welfare/economics/statistics & numerical data
Socioeconomic Factors
Statistics as Topic
Young Adult
Figure
Reference
-
1. Onakpoya IJ, Heneghan CJ, Aronson JK. Delays in the post-marketing withdrawal of drugs to which deaths have been attributed: a systematic investigation and analysis. BMC Med. 2015; 13:26.2. Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol. 2003; 1:194–205.3. Dieppe PA, Ebrahim S, Martin RM, Jüni P. Lessons from the withdrawal of rofecoxib. BMJ. 2004; 329:867–868.4. Friedman MA, Woodcock J, Lumpkin MM, Shuren JE, Hass AE, Thompson LJ. The safety of newly approved medicines: do recent market removals mean there is a problem? JAMA. 1999; 281:1728–1734.5. Bakke OM, Manocchia M, de Abajo F, Kaitin KI, Lasagna L. Drug safety discontinuations in the United Kingdom, the United States, and Spain from 1974 through 1993: a regulatory perspective. Clin Pharmacol Ther. 1995; 58:108–117.6. UN Department of Economic and Social Affairs. Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments. Pharmaceuticals Eighth Issue. New York: United Nations Publication;2003.7. Fung M, Thornton A, Mybeck K, Wu JH, Hornbuckle K, Muniz E. Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets-1960 to 1999*. Drug Inf J. 2001; 35:293–317.8. Ninan B, Wertheimer AI. Withdrawing drugs in the US versus other countries. University of Minnesota, College of Pharmacy;2012. accessed on 9 June 2014. Available at http://purl.umn.edu/137111.9. Makuch RW, Shi R. Comparison of drug approvals in europe versus the United States: An analysis of discrepancies between drug products reviewed by EMA and FDA. Ther Innov Regul Sci. 2014; 48:362–366.10. Abraham J, Davis C. A comparative analysis of drug safety withdrawals in the UK and the US (1971-1992): implications for current regulatory thinking and policy. Soc Sci Med. 2005; 61:881–892.11. Coloma PM, Schuemie MJ, Trifirò G, Gini R, Herings R, Hippisley-Cox J, Mazzaglia G, Giaquinto C, Corrao G, Pedersen L, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011; 20:1–11.12. Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R. Developing the Sentinel System--a national resource for evidence development. N Engl J Med. 2011; 364:498–499.13. UN Department of Economic and Social Affairs. Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments: pharmaceuticals tenth issue. New York: United Nations Publication;2004.14. UN Department of Economic and Social Affairs. Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments: pharmaceuticals tenth issue. New York: United Nations Publication;2004.15. UN Department of Economic and Social Affairs. Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments: pharmaceuticals fourteenth issue. New York: United Nations Publication;2009.16. OECD. OECD Statistics. accessed on 1 December 2014. Available at http://www.oecd-ilibrary.org/statistics.17. World Bank. World Development Indicators 2010. accessed on 15 Febulary 2015. Available at http://data.worldbank.org/indicator.18. Nam SH, Kim YS, Ju YS. A study of composite index on health and welfare. Seoul: Korea Institute for Health and Social Affairs;2012.19. Lim KJ, Lim SM, Seo KH. Analysis of the OECD Health Data 2012. Seoul: Research Institute for Healthcare Policy;2013.20. Kim L, Kim JA, Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health. 2014; 36:e2014008.21. R Development Core Team. R: a language and environment for statistical computing. Vienna: Austria R Foundation for Statistical Computing;2010.22. OECD. Life expectancy at birth and GDP per capita, 2011 (or nearest year). Health at a Glance. Paris: OECD Publishing;2013.23. Yang S, Khang YH, Harper S, Davey Smith G, Leon DA, Lynch J. Understanding the rapid increase in life expectancy in South Korea. Am J Public Health. 2010; 100:896–903.24. Hall W, Doran C, Degenhardt L, Shepard D. Illicit opiate abuse. In : Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, editors. Disease control priorities in developing countries. 2nd ed. Washington DC: World Bank Publications;2006. p. 907–932.25. Korea Food and Drug Administration. Dear healthcare professional letter: aprotinin. 2008. accessed on 9 June 2014. Available at http://drug.mfds.go.kr/html/articleLinkBody.jsp?p_menuId=0602&p_sub_menuId=060201&p_no=111.26. Choi D, Choi M, Ko A. Current status of pharmaceutical safety management in Korea. J Korean Med Assoc. 2012; 55:827–834.27. Ratanawijitrasin S, Wondemagegnehu E. Effective drug regulation: a multicountry study. Geneva, Switzerland: World Health Organization;2002.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Socioeconomic inequalities in health status in Korea
- Implementation status and future plans of Sustainable Development Goals in the health sector in Korea
- Analysis for the Impact of Adulthood and Childhood Socioeconomic Positions and Intergenerational Social Mobility on Adulthood Health
- A Review on Socioeconomic Position Indicators in Health Inequality Research
- Prescription of Gastric Acid Secretion Inhibitors before and after the Withdrawal of Ranitidine