J Periodontal Implant Sci.
2016 Aug;46(4):244-253. 10.5051/jpis.2016.46.4.244.
Four-week histologic evaluation of grafted calvarial defects with adjunctive hyperbaric oxygen therapy in rats
- Affiliations
-
- 1Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. kst72@snu.ac.kr
- 2Division of Anatomy and Developmental Biology, Department of Oral Biology, Human Identification Research Center, BK21 PLUS Project, Yonsei University College of Dentistry, Seoul, Korea.
- 3Department of Oral and Maxillofacial Surgery, Wonkwang University College of Dentistry, Iksan, Korea.
- KMID: 2350025
- DOI: http://doi.org/10.5051/jpis.2016.46.4.244
Abstract
- PURPOSE
The aim of this study was to characterize the healing in the grafted calvarial defects of rats after adjunctive hyperbaric oxygen therapy.
METHODS
Twenty-eight male Sprague-Dawley rats (body weight, 250-300 g) were randomly divided into two treatment groups: with hyperbaric oxygen therapy (HBO; n=14) and without HBO (NHBO; n=14). Each group was further subdivided according to the bone substitute applied: biphasic calcium phosphate (BCP; n=7) and surface-modified BCP (mBCP; n=7). The mBCP comprised BCP coated with Escherichia-coli-derived recombinant human bone morphogenetic protein-2 (ErhBMP-2) and epigallocatechin-3-gallate (EGCG). Two symmetrical circular defects (6-mm diameter) were created in the right and left parietal bones of each animal. One defect was assigned as a control defect and received no bone substitute, while the other defect was filled with either BCP or mBCP. The animals were allowed to heal for 4 weeks, during which those in the HBO group underwent 5 sessions of HBO. At 4 weeks, the animals were sacrificed, and the defects were harvested for histologic and histomorphometric analysis.
RESULTS
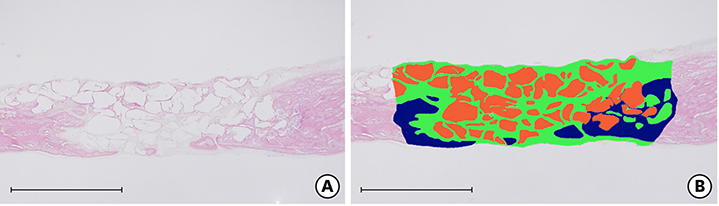
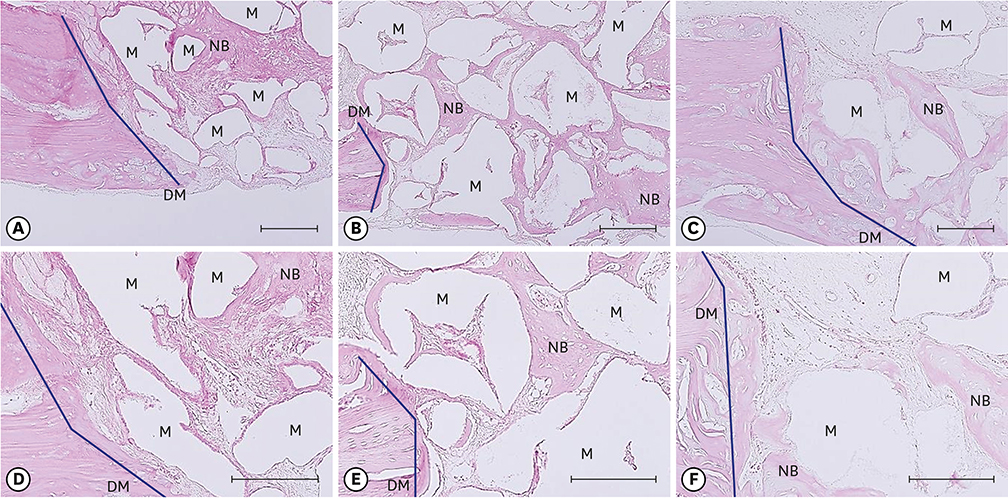
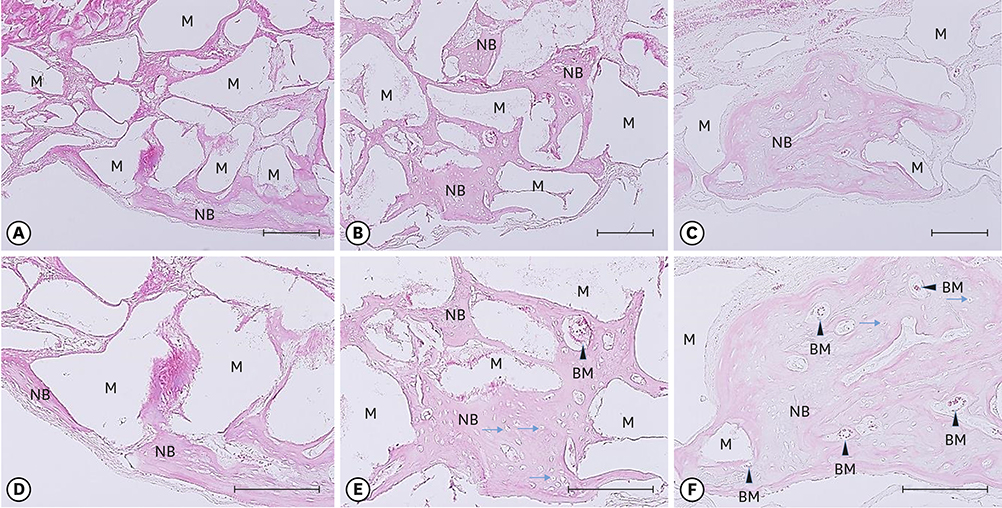
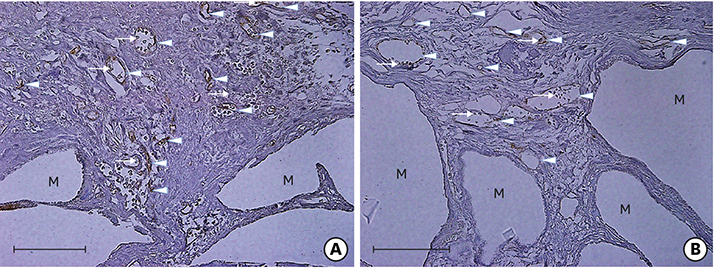
Well-maintained space was found in the grafted groups. Woven bone connected to and away from the defect margin was formed. More angiogenesis was found with HBO and EGCG/BMP-2 (P<0.05). None of the defects achieved complete defect closure. Increased new bone formation with HBO or EGCG/BMP-2 was evident in histologic evaluation, but it did not reach statistical significance in histometric analysis. A synergic effect between HBO and EGCG/BMP-2 was not found.
CONCLUSIONS
Within the limitations of this study, the present findings indicate that adjunctive HBO and EGCG/BMP-2 could be beneficial for new bone formation in rat calvarial defects.
MeSH Terms
Figure
Cited by 1 articles
-
Eight-week healing of grafted calvarial bone defects with hyperbaric oxygen therapy in rats
Seo-Eun Oh, Kyung-Seok Hu, Sungtae Kim
J Periodontal Implant Sci. 2019;49(4):228-236. doi: 10.5051/jpis.2019.49.4.228.
Reference
-
1. Hopf HW, Gibson JJ, Angeles AP, Constant JS, Feng JJ, Rollins MD, et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005; 13:558–564.
Article2. Howard MA, Asmis R, Evans KK, Mustoe TA. Oxygen and wound care: a review of current therapeutic modalities and future direction. Wound Repair Regen. 2013; 21:503–511.
Article3. Milovanova TN, Bhopale VM, Sorokina EM, Moore JS, Hunt TK, Hauer-Jensen M, et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. J Appl Physiol (1985). 2009; 106:711–728.
Article4. Jan AM, Sándor GK, Iera D, Mhawi A, Peel S, Evans AW, et al. Hyperbaric oxygen results in an increase in rabbit calvarial critical sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:144–149.
Article5. Fok TC, Jan A, Peel SA, Evans AW, Clokie CM, Sándor GK. Hyperbaric oxygen results in increased vascular endothelial growth factor (VEGF) protein expression in rabbit calvarial critical-sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 105:417–422.
Article6. Jan A, Sándor GK, Brkovic BB, Peel S, Evans AW, Clokie CM. Effect of hyperbaric oxygen on grafted and nongrafted calvarial critical-sized defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:157–163.
Article7. Muhonen A, Muhonen J, Lindholm TC, Minn H, Klossner J, Kulmala J, et al. Osteodistraction of a previously irradiated mandible with or without adjunctive hyperbaric oxygenation: an experimental study in rabbits. Int J Oral Maxillofac Surg. 2002; 31:519–524.
Article8. Muhonen A, Haaparanta M, Grönroos T, Bergman J, Knuuti J, Hinkka S, et al. Osteoblastic activity and neoangiogenesis in distracted bone of irradiated rabbit mandible with or without hyperbaric oxygen treatment. Int J Oral Maxillofac Surg. 2004; 33:173–178.
Article9. Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006; 3:49–57.
Article10. Palti A, Hoch T. A concept for the treatment of various dental bone defects. Implant Dent. 2002; 11:73–78.
Article11. Choi H, Park NJ, Jamiyandorj O, Choi KH, Hong MH, Oh S, et al. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with two concentrations of expressed recombinant human bone morphogenetic protein 2. J Periodontal Implant Sci. 2012; 42:119–126.
Article12. Kim MS, Kwon JY, Lee JS, Song JS, Choi SH, Jung UW. Low-dose recombinant human bone morphogenetic protein-2 to enhance the osteogenic potential of the Schneiderian membrane in the early healing phase: in vitro and in vivo studies. J Oral Maxillofac Surg. 2014; 72:1480–1494.
Article13. Choi H, Park NJ, Jamiyandorj O, Hong MH, Oh S, Park YB, et al. Improvement of osteogenic potential of biphasic calcium phosphate bone substitute coated with synthetic cell binding peptide sequences. J Periodontal Implant Sci. 2012; 42:166–172.
Article14. Shin YS, Seo JY, Oh SH, Kim JH, Kim ST, Park YB, et al. The effects of ErhBMP-2-/EGCG-coated BCP bone substitute on dehiscence around dental implants in dogs. Oral Dis. 2014; 20:281–287.
Article15. Jin P, Wu H, Xu G, Zheng L, Zhao J. Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: an in vitro study. Cell Tissue Res. 2014; 356:381–390.
Article16. Oka Y, Iwai S, Amano H, Irie Y, Yatomi K, Ryu K, et al. Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci. 2012; 118:55–64.
Article17. Sirin Y, Olgac V, Dogru-Abbasoglu S, Tapul L, Aktas S, Soley S. The influence of hyperbaric oxygen treatment on the healing of experimental defects filled with different bone graft substitutes. Int J Med Sci. 2011; 8:114–125.
Article18. Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005; 19:665–667.
Article19. Wang DS, Miura M, Demura H, Sato K. Anabolic effects of 1,25-dihydroxyvitamin D3 on osteoblasts are enhanced by vascular endothelial growth factor produced by osteoblasts and by growth factors produced by endothelial cells. Endocrinology. 1997; 138:2953–2962.
Article20. Bai Y, Leng Y, Yin G, Pu X, Huang Z, Liao X, et al. Effects of combinations of BMP-2 with FGF-2 and/or VEGF on HUVECs angiogenesis in vitro and CAM angiogenesis in vivo. Cell Tissue Res. 2014; 356:109–121.
Article21. Cai WX, Zheng LW, Li CL, Ma L, Ehrbar M, Weber FE, et al. Effect of different rhBMP-2 and TG-VEGF ratios on the formation of heterotopic bone and neovessels. Biomed Res Int. 2014; 2014:571510.
Article22. Lysdahl H, Baatrup A, Foldager CB, Bünger C. Preconditioning human mesenchymal stem cells with a low concentration of BMP2 stimulates proliferation and osteogenic differentiation in vitro. Biores Open Access. 2014; 3:278–285.
Article23. Park JS, Kim MH, Chang HJ, Kim KM, Kim SM, Shin BA, et al. Epigallocatechin-3-gallate inhibits the PDGF-induced VEGF expression in human vascular smooth muscle cells via blocking PDGF receptor and Erk-1/2. Int J Oncol. 2006; 29:1247–1252.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adjunctive hyperbaric oxygen therapy for irradiated rat calvarial defects
- Eight-week healing of grafted calvarial bone defects with hyperbaric oxygen therapy in rats
- Treatment of radiation-induced cystitis with hyperbaric oxygen
- Histologic Study on Healing after Implantation of several Bone Substitutes in Rat Calvarial Defects
- A Basic Survey for Regional Capability of Hyperbaric Oxygen Therapy to Multiple Fire Victims