Yonsei Med J.
2015 Nov;56(6):1714-1720. 10.3349/ymj.2015.56.6.1714.
Pathological Evaluation of Radiation-Induced Vascular Lesions of the Brain: Distinct from De Novo Cavernous Hemangioma
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. paxco@yuhs.ac
- 2Department of Radiology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2345904
- DOI: http://doi.org/10.3349/ymj.2015.56.6.1714
Abstract
- PURPOSE
We aimed to evaluate the histologic and radiologic findings of vascular lesions after stereotactic radiosurgery (SRS) categorized as radiation-induced cavernous hemangioma (RICH).
MATERIALS AND METHODS
Among 89 patients who underwent neurosurgery for cavernous hemangioma, eight RICHs from 7 patients and 10 de novo CHs from 10 patients were selected for histopathological and radiological comparison.
RESULTS
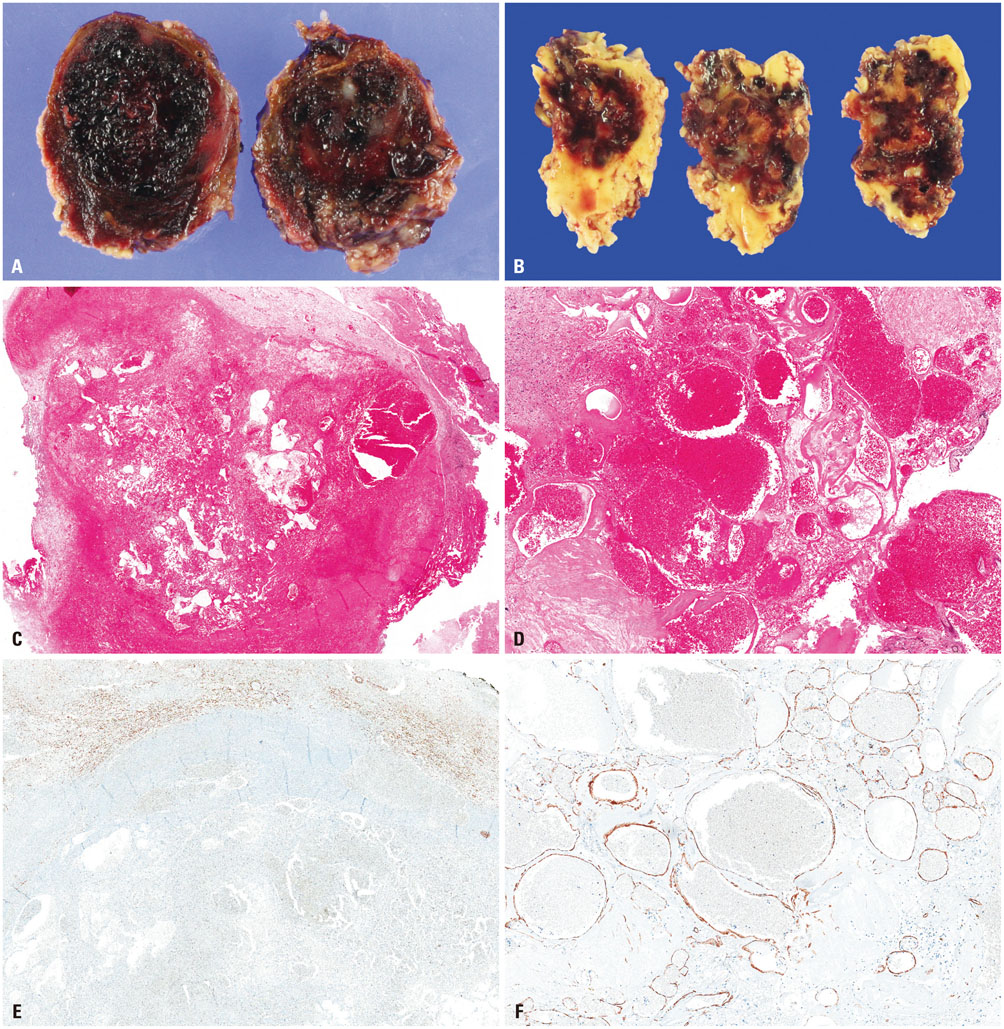
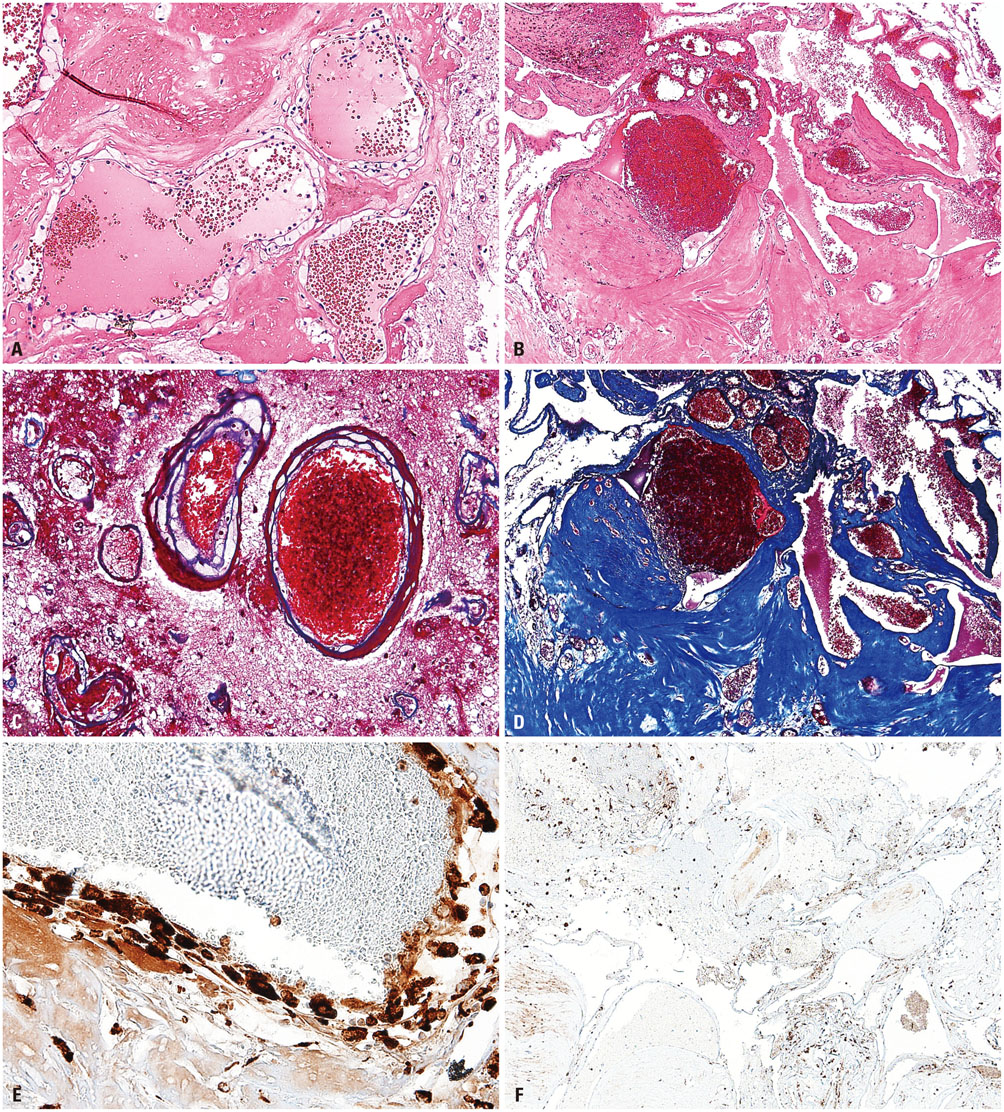
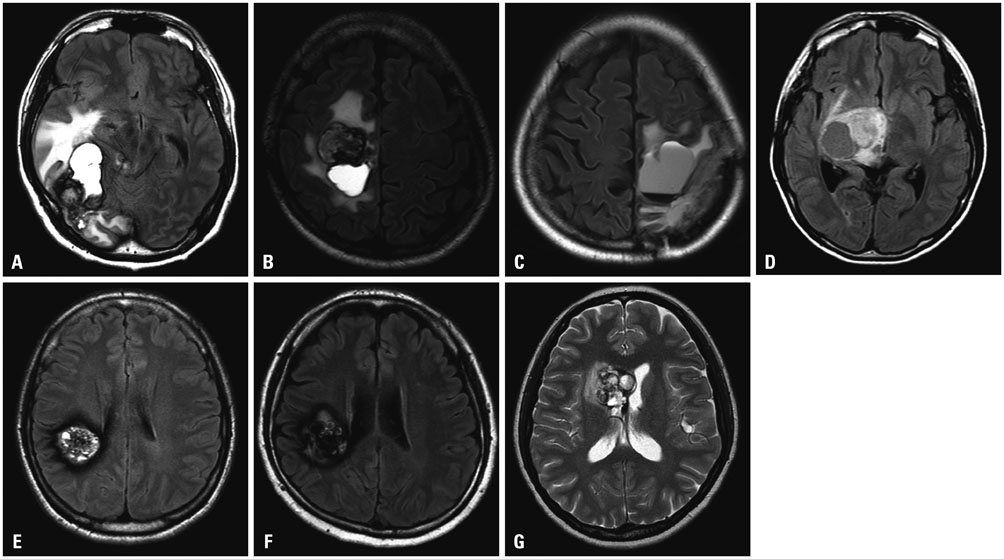
Histologically, RICHs showed hematoma-like gross appearance. Microscopically, RICH exhibited a hematoma-like area accompanied by proliferation of thin-walled vasculature with fibrin deposits and infiltrating foamy macrophages. In contrast, CHs demonstrated localized malformed vasculature containing fresh and old clotted blood on gross examination. Typically, CHs consisted of thick, ectatic hyalinized vessels lined by endothelium under a light microscope. Magnetic resonance imaging of RICHs revealed some overlapping but distinct features with CHs, including enhancing cystic and solid components with absence or incomplete popcorn-like appearance and partial hemosiderin rims.
CONCLUSION
Together with histologic and radiologic findings, RICH may result from blood-filled space after tissue destruction by SRS, accompanied with radiation-induced reactive changes rather than vascular malformation. Thus, the term "RICH" would be inappropriate, because it is more likely to be an inactive organizing hematoma rather than proliferation of malformed vasculature.
Keyword
MeSH Terms
Figure
Reference
-
1. Del Curling O Jr, Kelly DL Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991; 75:702–708.
Article2. Hegde AN, Mohan S, Lim CC. CNS cavernous haemangioma: "popcorn" in the brain and spinal cord. Clin Radiol. 2012; 67:380–388.
Article3. Mindea SA, Yang BP, Shenkar R, Bendok B, Batjer HH, Awad IA. Cerebral cavernous malformations: clinical insights from genetic studies. Neurosurg Focus. 2006; 21:e1.
Article4. Koike T, Yanagimachi N, Ishiguro H, Yabe H, Yabe M, Morimoto T, et al. High incidence of radiation-induced cavernous hemangioma in long-term survivors who underwent hematopoietic stem cell transplantation with radiation therapy during childhood or adolescence. Biol Blood Marrow Transplant. 2012; 18:1090–1098.
Article5. Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 2006; 21:e4.
Article6. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951; 102:316–319.7. Salvetti DJ, Nagaraja TG, Levy C, Xu Z, Sheehan J. Gamma Knife surgery for the treatment of patients with asymptomatic meningiomas. J Neurosurg. 2013; 119:487–493.
Article8. Yianni J, Rowe J, Khandanpour N, Nagy G, Hoggard N, Radatz M, et al. Stereotactic radiosurgery for pineal tumours. Br J Neurosurg. 2012; 26:361–366.
Article9. Xu Z, Elsharkawy M, Schlesinger D, Sheehan J. Gamma knife radiosurgery for resectable brain metastasis. World Neurosurg. 2013; 80:351–358.
Article10. Salvetti DJ, Nagaraja TG, McNeill IT, Xu Z, Sheehan J. Gamma Knife surgery for the treatment of 5 to 15 metastases to the brain: clinical article. J Neurosurg. 2013; 118:1250–1257.
Article11. Friedman WA. Stereotactic radiosurgery of intracranial arteriovenous malformations. Neurosurg Clin N Am. 2013; 24:561–574.
Article12. Bradac O, Charvat F, Benes V. Treatment for brain arteriovenous malformation in the 1998-2011 period and review of the literature. Acta Neurochir (Wien). 2013; 155:199–209.
Article13. Matsumoto H, Takeda T, Kohno K, Yamaguchi Y, Kohno K, Takechi A, et al. Delayed hemorrhage from completely obliterated arteriovenous malformation after gamma knife radiosurgery. Neurol Med Chir (Tokyo). 2006; 46:186–190.
Article14. Keezer MR, Del Maestro R. Radiation-induced cavernous hemangiomas: case report and literature review. Can J Neurol Sci. 2009; 36:303–310.
Article15. Heckl S, Aschoff A, Kunze S. Radiation-induced cavernous hemangiomas of the brain: a late effect predominantly in children. Cancer. 2002; 94:3285–3291.
Article16. Koike S, Aida N, Hata M, Fujita K, Ozawa Y, Inoue T. Asymptomatic radiation-induced telangiectasia in children after cranial irradiation: frequency, latency, and dose relation. Radiology. 2004; 230:93–99.
Article17. Gaensler EH, Dillon WP, Edwards MS, Larson DA, Rosenau W, Wilson CB. Radiation-induced telangiectasia in the brain simulates cryptic vascular malformations at MR imaging. Radiology. 1994; 193:629–636.
Article18. Humpl T, Brühl K, Bohl J, Schwarz M, Stoeter P, Gutjahr P. Cerebral haemorrhage in long-term survivors of childhood acute lymphoblastic leukaemia. Eur J Pediatr. 1997; 156:367–370.
Article19. Larson JJ, Ball WS, Bove KE, Crone KR, Tew JM Jr. Formation of intracerebral cavernous malformations after radiation treatment for central nervous system neoplasia in children. J Neurosurg. 1998; 88:51–56.
Article20. Baumgartner JE, Ater JL, Ha CS, Kuttesch JF, Leeds NE, Fuller GN, et al. Pathologically proven cavernous angiomas of the brain following radiation therapy for pediatric brain tumors. Pediatr Neurosurg. 2003; 39:201–207.
Article21. Jain R, Robertson PL, Gandhi D, Gujar SK, Muraszko KM, Gebarski S. Radiation-induced cavernomas of the brain. AJNR Am J Neuroradiol. 2005; 26:1158–1162.22. Furuse M, Miyatake SI, Kuroiwa T. Cavernous malformation after radiation therapy for astrocytoma in adult patients: report of 2 cases. Acta Neurochir (Wien). 2005; 147:1097–1101.
Article23. Sasagawa Y, Akai T, Itou S, Iizuka H. Gamma knife radiosurgery-induced cavernous hemangioma: case report. Neurosurgery. 2009; 64:E1006–E1007.24. Kiliç T, Pamir MN, Küllü S, Eren F, Ozek MM, Black PM. Expression of structural proteins and angiogenic factors in cerebrovascular anomalies. Neurosurgery. 2000; 46:1179–1191.
Article25. Tsao MN, Li YQ, Lu G, Xu Y, Wong CS. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999; 58:1051–1060.
Article26. Freeman CR, Souhami L, Caron JL, Villemure JG, Olivier A, Montes J, et al. Stereotactic external beam irradiation in previously untreated brain tumors in children and adolescents. Med Pediatr Oncol. 1994; 22:173–180.
Article27. El Ahmadieh TY, Aoun SG, Bendok BR, Batjer HH. Management of brainstem cavernous malformations. Curr Treat Options Cardiovasc Med. 2012; 14:237–251.
Article28. Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol. 1991; 12:45–62.29. Martins AN, Johnston JS, Henry JM, Stoffel TJ, Di Chiro G. Delayed radiation necrosis of the brain. J Neurosurg. 1977; 47:336–345.
Article30. Schiffer D, Giordana MT, Soffietti R, Tarenzi L, Milani R, Vasario E, et al. Radio- and chemotherapy of malignant gliomas. Pathological changes in the normal nervous tissue. Acta Neurochir (Wien). 1981; 58:37–58.
Article31. Reinhold HS, Hopewell JW. Late changes in the architecture of blood vessels of the rat brain after irradiation. Br J Radiol. 1980; 53:693–696.
Article32. Rottenberg DA, Chernik NL, Deck MD, Ellis F, Posner JB. Cerebral necrosis following radiotherapy of extracranial neoplasms. Ann Neurol. 1977; 1:339–357.
Article33. Lampert PW, Davis RL. Delayed effects of radiation on the human central nervous system; "Early" and "Late" delayed reactions. Neurology. 1964; 14:912–917.
Article34. Park YS, Kim SH, Chang JH, Chang JW, Park YG. Radiosurgery for radiosurgery-induced cavernous malformation. World Neurosurg. 2011; 75:94–98.
Article35. Vinchon M, Leblond P, Caron S, Delestret I, Baroncini M, Coche B. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Childs Nerv Syst. 2011; 27:445–453.
Article36. Faraci M, Morana G, Bagnasco F, Barra S, Polo P, Hanau G, et al. Magnetic resonance imaging in childhood leukemia survivors treated with cranial radiotherapy: a cross sectional, single center study. Pediatr Blood Cancer. 2011; 57:240–246.
Article37. Duhem R, Vinchon M, Leblond P, Soto-Ares G, Dhellemmes P. Cavernous malformations after cerebral irradiation during childhood: report of nine cases. Childs Nerv Syst. 2005; 21:922–925.
Article38. Tung GA, Noren G, Rogg JM, Jackson IM. MR imaging of pituitary adenomas after gamma knife stereotactic radiosurgery. AJR Am J Roentgenol. 2001; 177:919–924.
Article39. Chernov MF, Ono Y, Abe K, Usukura M, Hayashi M, Izawa M, et al. Differentiation of tumor progression and radiation-induced effects after intracranial radiosurgery. Acta Neurochir Suppl. 2013; 116:193–210.
Article40. Sharpton SR, Oermann EK, Moore DT, Schreiber E, Hoffman R, Morris DE, et al. The volumetric response of brain metastases after stereotactic radiosurgery and its post-treatment implications. Neurosurgery. 2014; 74:9–15.
Article