Korean Circ J.
2016 Mar;46(2):135-144. 10.4070/kcj.2016.46.2.135.
Hypertriglyceridemia and Cardiovascular Diseases: Revisited
- Affiliations
-
- 1Department of Cardiology, Gachon University Gil Medical Center, Incheon, Korea. kwangk@gilhospital.com
- 2Department of Cardiology, South Australian Health and Medical Research Institute, University of Adelaide, Adelaide, Australia.
- 3Department of Cardiovascular Medinine, Hokko Memorial Clinic, Sapporo, Japan.
- 4Department of Epidemiology, Beijing Institute of Heart, Lung and Blood Vessel Diseases, Beijing An Zhen Hospital, Capital Medical University, Beijing, China.
- KMID: 2344463
- DOI: http://doi.org/10.4070/kcj.2016.46.2.135
Abstract
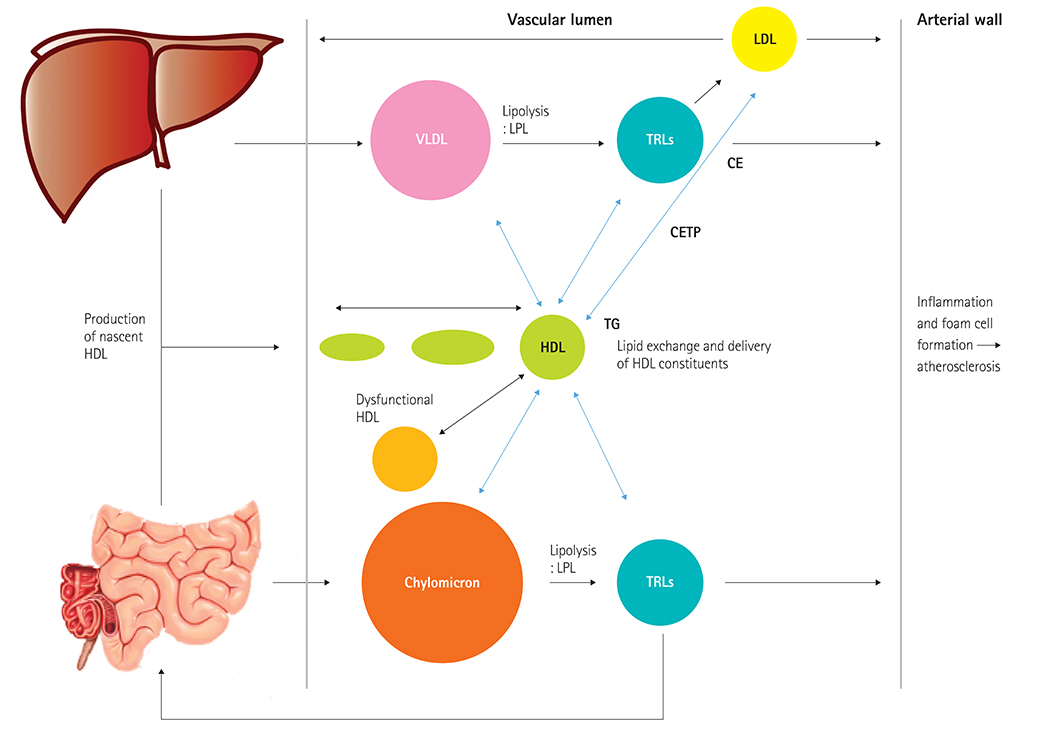
- Residual cardiovascular risk and failure of high density lipoprotein cholesterol raising treatment have refocused interest on targeting hypertriglyceridemia. Hypertriglyceridemia, triglyceride-rich lipoproteins, and remnant cholesterol have demonstrated to be important risk factors for cardiovascular disease; this has been demonstrated in experimental, genetic, and epidemiological studies. Fibrates can reduce cardiovascular event rates with or without statins. High dose omega-3 fatty acids continue to be evaluated and new specialized targeting treatment modulating triglyceride pathways, such as inhibition of apolipoprotein C-III and angiopoietin-like proteins, are being tested with regard to their effects on lipid profiles and cardiovascular outcomes. In this review, we will discuss the role of hypertriglyceridemia, triglyceride-rich lipoproteins and remnant cholesterol on cardiovascular disease, and the potential implications for treatment stargeting hypertriglyceridemia.
MeSH Terms
-
Apolipoprotein C-III
Cardiovascular Diseases*
Cholesterol
Cholesterol, HDL
Epidemiologic Studies
Fatty Acids, Omega-3
Fibric Acids
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Hypertriglyceridemia*
Lipoproteins
Risk Factors
Triglycerides
Apolipoprotein C-III
Cholesterol
Cholesterol, HDL
Fatty Acids, Omega-3
Fibric Acids
Lipoproteins
Figure
Reference
-
1. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014; 63(25 Pt B):2889–2934.2. Mora S, Wenger NK, DeMicco DA, et al. Determinants of residual risk in secondary prevention patients treated with high- versus low-dose statin therapy: the treating to new targets (TNT) study. Circulation. 2012; 125:1979–1987.3. Lim S, Park YM, Sakuma I, Koh KK. How to control residual cardiovascular risk despite statin treatment: Focusing on HDL–cholesterol. Int J Cardiol. 2013; 166:8–14.4. Koh KK. How to control residual risk during statin era? J Am Coll Cardiol. 2015; 66:1848.5. Mora S, Caulfield MP, Wohlgemuth J, et al. Atherogenic lipoprotein subfractions determined by ion mobility and first cardiovascular events after random allocation to high-intensity statin or placebo: the JUPITER trial. Circulation. 2015; 132:2220–2229.6. Lee MH, Kim HC, Ahn SV, et al. Prevalence of dyslipidemia among Korean adults: Korea National Health and Nutrition Survey 1998-2005. Diabetes Metab J. 2012; 36:43–55.7. Kim K. Distribution of blood cholesterol profile in untreated Korean population. Korean Circ J. 2015; 45:108–109.8. Park JH, Lee MH, Shim JS, et al. Effects of age, sex, and menopausal status on blood cholesterol profile in the Korean population. Korean Circ J. 2015; 45:141–148.9. Ren J, Grundy SM, Liu J, et al. Long-term coronary heart disease risk associated with very-low-density lipoprotein cholesterol in Chinese: the results of a 15-Year Chinese Multi-Provincial Cohort Study (CMCS). Atherosclerosis. 2010; 211:327–332.10. Lim S, Shin H, Song JH, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011; 34:1323–1328.11. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014; 384:626–635.12. Nordestgaard BG, Wootton R, Lewis B. Selective retention of VLDL, IDL, and LDL in the arterial intima of genetically hyperlipidemic rabbits in vivo. Molecular size as a determinant of fractional loss from the intima-inner media. Arterioscler Thromb Vasc Biol. 1995; 15:534–542.13. Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol. 2011; 31:1716–1725.14. Proctor SD, Vine DF, Mamo JC. Arterial retention of apolipoprotein B(48)- and B(100)-containing lipoproteins in atherogenesis. Curr Opin Lipidol. 2002; 13:461–470.15. Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011; 32:1345–1361.16. Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013; 61:427–436.17. Hegele RA, Ginsberg HN, Chapman MJ, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014; 2:655–666.18. Rapp JH, Lespine A, Hamilton RL, et al. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb. 1994; 14:1767–1774.19. Alaupovic P, Mack WJ, Knight-Gibson C, Hodis HN. The role of triglyceride-rich lipoprotein families in the progression of atherosclerotic lesions as determined by sequential coronary angiography from a controlled clinical trial. Arterioscler Thromb Vasc Biol. 1997; 17:715–722.20. Zheng XY, Liu L. Remnant-like lipoprotein particles impair endothelial function: direct and indirect effects on nitric oxide synthase. J Lipid Res. 2007; 48:1673–1680.21. Alipour A, van Oostrom AJ, Izraeljan A, et al. Leukocyte activation by triglyceride-rich lipoproteins. Arterioscler Thromb Vasc Biol. 2008; 28:792–797.22. Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009; 50:204–213.23. Moyer MP, Tracy RP, Tracy PB, van't Veer Cvt, Sparks CE, Mann KG. Plasma lipoproteins support prothrombinase and other procoagulant enzymatic complexes. Arterioscler Thromb Vasc Biol. 1998; 18:458–465.24. Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med. 2000; 342:1792–1801.25. Patel A, Barzi F, Jamrozik K, et al. Serum triglycerides as a risk factor for cardiovascular diseases in the Asia-Pacific Region. Circulation. 2004; 110:2678–2686.26. Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease 10,158 incident cases among 262,525 participants in 29 western prospective studies. Circulation. 2007; 115:450–458.27. Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007; 298:299–308.28. Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007; 298:309–316.29. Langsted A, Freiberg J, Tybjærg-Hansen A, Schnohr P, Jensen GB, Nordestgaard B. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med. 2011; 270:65–75.30. Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013; 34:1826–1833.31. Emerging Risk Factors Collaboration. Di Angelantoni E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009; 302:1993–2000.32. Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014; 64:2525–2540.33. Schwartz GG, Abt M, Bao W, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015; 65:2267–2275.34. Jackson KG, Poppitt SD, Minihane AM. Postprandial lipemia and cardiovascular disease risk: interrelationships between dietary, physiological and genetic determinants. Atherosclerosis. 2012; 220:22–33.35. Rip J, Nierman MC, Ross CJ, et al. Lipoprotein lipase S447X a naturally occurring gain-of-function mutation. Arterioscler Thromb Vasc Biol. 2006; 26:1236–1245.36. Humphries SE, Nicaud V, Margalef J, Tiret L, Talmud PJ. Lipoprotein lipase gene variation is associated with a paternal history of premature coronary artery disease and fasting and postprandial plasma triglycerides: the European Atherosclerosis Research Study (EARS). Arterioscler Thromb Vasc Biol. 1998; 18:526–534.37. Wittrup HH, Tybjærg-Hansen A, Nordestgaard BG. Lipoprotein lipase mutations, plasma lipids and lipoproteins, and risk of ischemic heart disease. A meta-analysis. Circulation. 1999; 99:2901–2907.38. Henderson HE, Kastelein JJ, Zwinderman AH, et al. Lipoprotein lipase activity is decreased in a large cohort of patients with coronary artery disease and is associated with changes in lipids and lipoproteins. J Lipid Res. 1999; 40:735–743.39. Lettre G, Palmer CD, Young T, et al. Genome-wide association study of coronary heart disease and its risk factors in 8,090 African Americans: the NHLBI CARe project. PLoS Genet. 2011; 7:e1001300.40. Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010; 466:707–713.41. Waterworth DM, Ricketts SL, Song K, et al. Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol. 2010; 30:2264–2276.42. Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013; 45:1345–1352.43. Khetarpal SA, Rader DJ. Triglyceride-rich lipoproteins and coronary artery disease risk: new insights from human genetics. Arterioscler Thromb Vasc Biol. 2015; 35:e3–e9.44. Ooi EM, Barrett PH, Chan DC, Watts GF. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin Sci (Lond). 2008; 114:611–624.45. Gaudet D, Alexander VJ, Baker BF, et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med. 2015; 373:438–447.46. Kawakami A, Osaka M, Tani M, et al. Apolipoprotein CIII links hyperlipidemia with vascular endothelial cell dysfunction. Circulation. 2008; 118:731–742.47. Abe Y, Kawakami A, Osaka M, et al. Apolipoprotein CIII induces monocyte chemoattractant protein-1 and interleukin 6 expression via Toll-like receptor 2 pathway in mouse adipocytes. Arterioscler Thromb Vasc Biol. 2010; 30:2242–2248.48. Qamar A, Khetarpal SA, Khera AV, Qasim A, Rader DJ, Reilly MP. Plasma apolipoprotein C-III levels, triglycerides, and coronary artery calcification in type 2 diabetics. Arterioscler Thromb Vasc Biol. 2015; 35:1880–1888.49. TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. Crosby J, Peloso GM, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014; 371:22–31.50. Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014; 371:32–41.51. Pennacchio LA, Olivier M, Hubacek JA, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001; 294:169–173.52. Tang Y, Sun P, Guo D, et al. A genetic variant c.553G > T in the apolipoprotein A5 gene is associated with an increased risk of coronary artery disease and altered triglyceride levels in a Chinese population. Atherosclerosis. 2006; 185:433–437.53. Johansen CT, Wang J, Lanktree MB, et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet. 2010; 42:684–687.54. Soufi M, Sattler AM, Kurt B, Schaefer JR. Mutation screening of the APOA5 gene in subjects with coronary artery disease. J Investig Med. 2012; 60:1015–1019.55. Do R, Stitziel NO, Won HH, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015; 518:102–106.56. Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors collaboration. Sarwar N, Sandhu MS, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010; 375:1634–1639.57. Han SH, Quon MJ, Koh KK. Beneficial vascular and metabolic effects of peroxisome proliferator-activated receptor-alpha activators. Hypertension. 2005; 46:1086–1092.58. Han SH, Oh PC, Lim S, Eckel RH, Koh KK. Comparative cardiometabolic effects of fibrates and omega-3 fatty acids. Int J Cardiol. 2013; 167:2404–2411.59. Koh KK, Ahn JY, Han SH, et al. Effects of fenofibrate on lipoproteins, vasomotor function, and serological markers of inflammation, plaque stabilization, and hemostasis. Atherosclerosis. 2004; 174:379–383.60. Koh KK, Han SH, Quon MJ, Ahn JY, Shin EK. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care. 2005; 28:1419–1424.61. Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010; 375:1875–1884.62. Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010; 363:692–694. author reply 694-5.63. Taher TH, Dzavik V, Reteff EM, Pearson GJ, Woloschuk BL, Francis GA. Tolerability of statin-fibrate and statin-niacin combination therapy in dyslipidemic patients at high risk for cardiovascular events. Am J Cardiol. 2002; 89:390–394.64. Koh KK, Quon MJ, Han SH, et al. Additive beneficial effects of fenofibrate combined with atorvastatin in the treatment of combined hyperlipidemia. J Am Coll Cardiol. 2005; 45:1649–1653.65. ACCORD Study Group. Ginsberg HN, Elam MB, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362:1563–1574.66. Koh KK, Quon MJ, Shin KC, et al. Significant differential effects of omega-3 fatty acids and fenofibrate in patients with hypertriglyceridemia. Atherosclerosis. 2012; 220:537–544.67. Kromhout D, Giltay EJ, Geleijnse JM. Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010; 363:2015–2026.68. Kwak SM, Myung SK, Lee YJ, Seo HG. Korean Meta-analysis Study Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012; 172:686–694.69. Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr. 2009; 90:613–620.70. Djoussé L, Gaziano JM, Buring JE, Lee IM. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr. 2011; 93:143–150.71. Oh PC, Koh KK, Sakuma I, et al. Omega-3 fatty acid therapy dose-dependently and significantly decreased triglycerides and improved flow-mediated dilation, however, did not significantly improve insulin sensitivity in patients with hypertriglyceridemia. Int J Cardiol. 2014; 176:696–702.72. Rischio and Prevenzione Investigators. Efficacy of n-3 polyunsaturated fatty acids and feasibility of optimizing preventive strategies in patients at high cardiovascular risk: rationale, design and baseline characteristics of the Rischio and Prevenzione study, a large randomised trial in general practice. Trials. 2010; 11:68.73. Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012; 33:159–171.74. Chapman MJ, Redfern JS, McGovern ME, Giral P. Niacin and fibrates in atherogenic dyslipidemia: pharmacotherapy to reduce cardiovascular risk. Pharmacol Ther. 2010; 126:314–345.75. Clofibrate and niacin in coronary heart disease. JAMA. 1975; 231:360–381.76. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986; 8:1245–1255.77. HPS2-THRIVE Collaborative Group. Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high risk patients. N Engl J Med. 2014; 371:203–212.78. Crouse JR 3rd. Hypertriglyceridemia: a contraindication to the use of bile acid binding resins. Am J Med. 1987; 83:243–248.79. Chapman MJ, Le Goff W, Guerin M, Kontush A. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur Heart J. 2010; 31:149–164.80. Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007; 357:2109–2122.81. Schwartz GC, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. New Engl J Med. 2012; 367:2089–2099.82. Nicholls SJ, Lincoff AM, Barter PJ, et al. Assessment of the clinical effects of cholesteryl ester transfer protein inhibition with evacetrapib in patients at high-risk for vascular outcomes: rationale and design of the ACCELERATE trial. Am Heart J. 2015; 170:1061–1069.83. Korstanje R, Eriksson P, Samnegård A, et al. Locating Ath8, a locus for murine atherosclerosis susceptibility and testing several of its candidate genes in mice and humans. Atherosclerosis. 2004; 177:443–450.84. Hatsuda S, Shoji T, Shinohara K, et al. Association between plasma angiopoietin-like protein 3 and arterial wall thickness in healthy subjects. J Vasc Res. 2007; 44:61–66.85. Talmud PJ, Smart M, Presswood E, et al. ANGPTL4 E40K and T266M: effects on plasma triglyceride and HDL levels, postprandial responses, and CHD risk. Arterioscler Thromb Vasc Biol. 2008; 28:2319–2325.86. Sacks FM, Stanesa M, Hegele RA. Severe hypertriglyceridemia with pancreatitis: thirteen years' treatment with lomitapide. JAMA Intern Med. 2014; 174:443–447.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Hypertriglyceridemia for Prevention of Cardiovascular Diseases
- Hypertriglyceridemia: A Neglected Risk Factor for Ischemic Stroke?
- Abnormally low LDL-Cholesterol Level in Cholestasis or Hypertriglyceridemia
- Pharmacological treatment of hypertriglyceridemia
- Stressful life events and serum triglyceride levels: the Cardiovascular and Metabolic Diseases Etiology Research Center cohort in Korea