Cancer Res Treat.
2016 Jul;48(3):1110-1119. 10.4143/crt.2015.289.
Clinical Characteristics and Continued Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Administration in EGFR-mutated Non-Small Cell Lung Cancer with Skeletal Metastasis
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. jinkang@catholic.ac.kr
- 2Cancer Research Institute, The Catholic University of Korea, Seoul, Korea.
- 3Department of Radiation Oncology, The Catholic University of Korea, Seoul, Korea.
- 4Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Department of Radiology, ollege of Medicine, The Catholic University of Korea, Seoul, Korea.
- 6Department of Nuclear Medicine, ollege of Medicine, The Catholic University of Korea, Seoul, Korea.
- 7Department of Orthopedics, ollege of Medicine, The Catholic University of Korea, Seoul, Korea.
- 8Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2344085
- DOI: http://doi.org/10.4143/crt.2015.289
Abstract
- PURPOSE
The aim of this study was to analyze clinical characteristics of skeletal metastasis in epidermal growth factor receptor (EGFR) mutant non-small cell lung cancer (NSCLC) and treatment outcomes of continued EGFR tyrosine kinase inhibitor (TKI) therapy in patients presenting with skeletal metastasis progression.
MATERIALS AND METHODS
Of the 216 patients treated with EGFR-TKI for management of stage III-IV NSCLC between 2006 and 2012 in Seoul St. Mary's Hospital, 76 patients with confirmed EGFR-mutated NSCLC with skeletal metastases during therapy were analyzed retrospectively.
RESULTS
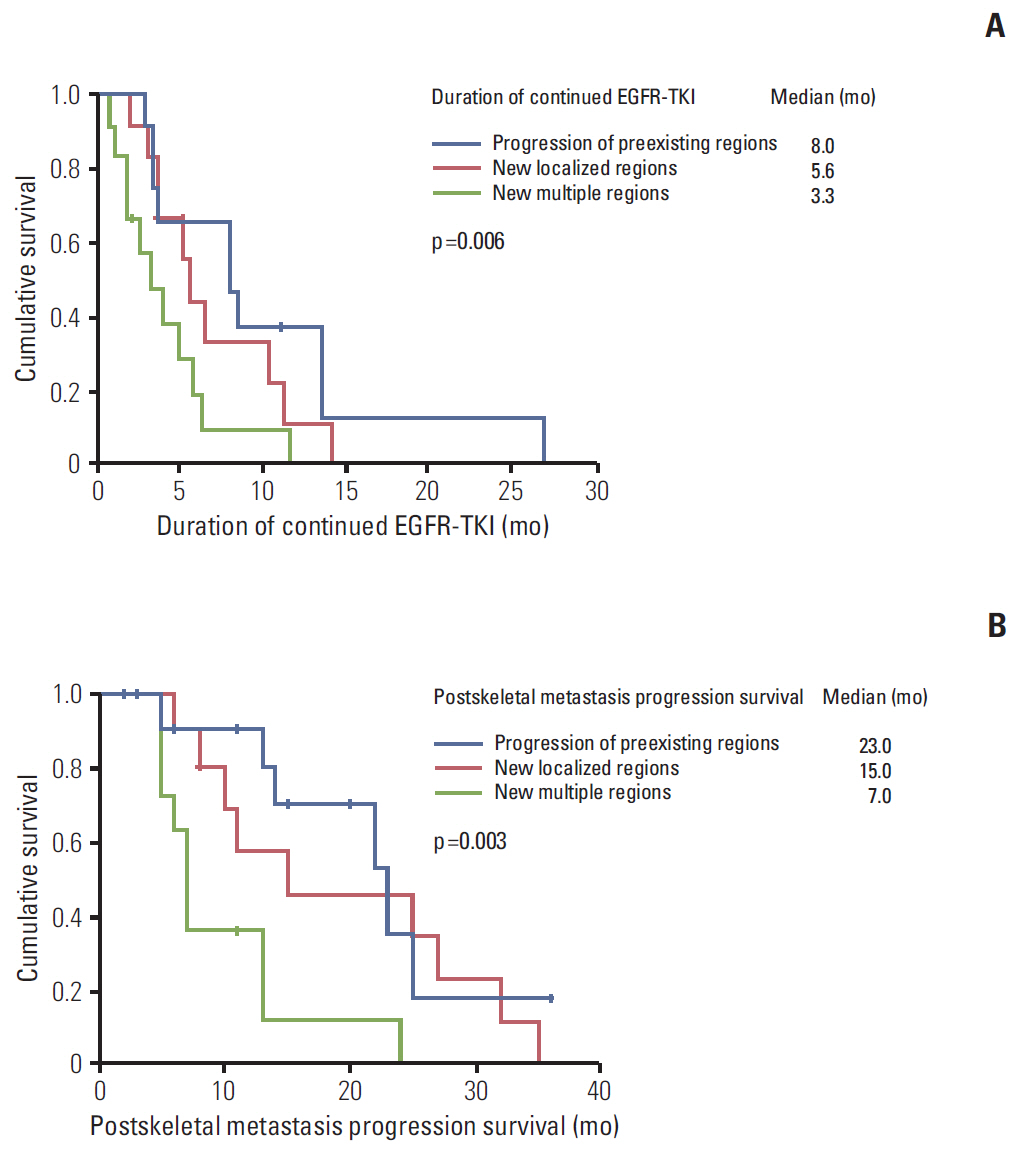
Of 76 patients with EGFR mutant lung cancer with skeletal metastasis, 37 patients developed first progressive disease (PD) in skeletal regions. EGFR-TKI was continued in these 37 patients after first PD in skeletal regions. Median time to first PD of skeletal regions was 8.9 months (95% confidence interval [CI], 4.8 to 13.0). Median time of continued EGFR-TKI after first PD of skeletal regions was 8.0 months (95% CI, 2.9 to 13.0) in patients with disease progression of preexisting regions, 5.6 months (95% CI, 4.5 to 6.7) in patients showing new localized regions, and 3.3 months (95% CI, 1.1 to 5.5) in patients with multiple new metastatic regions (p=0.006). Median time of postskeletal metastasis progression survival was 23.0 months (95% CI, 13.5 to 32.5), 15.0 months (95% CI, 3 to 34.7), and 7.0 months (95% CI, 6.0 to 8.0) (p=0.004) in the above described patient groups, respectively. Overall, seven patients (18.9%) had more than one episode of skeletal progression of disease without extraskeletal PD.
CONCLUSION
Continued EGFR-TKI treatment with adequate local treatment after progression of skeletal metastasis may be considered for patients who show disease progression in preexisting regions or local progression.
Keyword
MeSH Terms
-
Carcinoma, Non-Small-Cell Lung*
Disease Progression
Epidermal Growth Factor*
Humans
Lung Neoplasms
Neoplasm Metastasis*
Protein-Tyrosine Kinases
Receptor, Epidermal Growth Factor*
Response Evaluation Criteria in Solid Tumors
Retrospective Studies
Seoul
Epidermal Growth Factor
Protein-Tyrosine Kinases
Receptor, Epidermal Growth Factor
Figure
Reference
-
References
1. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010; 362:2380–8.
Article2. Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011; 17:6298–303.
Article3. Conforti F, Catania C, Toffalorio F, Duca M, Spitaleri G, Barberis M, et al. EGFR tyrosine kinase inhibitors beyond focal progression obtain a prolonged disease control in patients with advanced adenocarcinoma of the lung. Lung Cancer. 2013; 81:440–4.
Article4. Nishie K, Kawaguchi T, Tamiya A, Mimori T, Takeuchi N, Matsuda Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol. 2012; 7:1722–7.
Article5. Yang JJ, Chen HJ, Yan HH, Zhang XC, Zhou Q, Su J, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer. 2013; 79:33–9.
Article6. Coleman RE. Skeletal complications of malignancy. Cancer. 1997; 80(8 Suppl):1588–94.
Article7. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001; 27:165–76.
Article8. Bury T, Barreto A, Daenen F, Barthelemy N, Ghaye B, Rigo P. Fluorine-18 deoxyglucose positron emission tomography for the detection of bone metastases in patients with non-small cell lung cancer. Eur J Nucl Med. 1998; 25:1244–7.
Article9. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004; 350:2129–39.
Article10. Choi HS, Yoo IR, Park HL, Choi EK, Kim SH, Lee WH. Role of (1)(8)F-FDG PET/CT in differentiation of a benign lesion and metastasis on the ribs of cancer patients. Clin Imaging. 2014; 38:109–14.
Article11. Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010; 1:80–92.
Article12. Chao HS, Chang CP, Chiu CH, Chu LS, Chen YM, Tsai CM. Bone scan flare phenomenon in non-small-cell lung cancer patients treated with gefitinib. Clin Nucl Med. 2009; 34:346–9.
Article13. Hendriks LE, Smit EF, Vosse BA, Mellema WW, Heideman DA, Bootsma GP, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer. 2014; 84:86–91.
Article14. Scagliotti GV, Hirsh V, Siena S, Henry DH, Woll PJ, Manegold C, et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol. 2012; 7:1823–9.
Article15. Normanno N, Gullick WJ. Epidermal growth factor receptor tyrosine kinase inhibitors and bone metastases: different mechanisms of action for a novel therapeutic application? Endocr Relat Cancer. 2006; 13:3–6.
Article16. Inomata M, Shukuya T, Takahashi T, Ono A, Nakamura Y, Tsuya A, et al. Continuous administration of EGFR-TKIs following radiotherapy after disease progression in bone lesions for non-small cell lung cancer. Anticancer Res. 2011; 31:4519–23.17. Nishino M, Dahlberg SE, Cardarella S, Jackman DM, Rabin MS, Ramaiya NH, et al. Volumetric tumor growth in advanced non-small cell lung cancer patients with EGFR mutations during EGFR-tyrosine kinase inhibitor therapy: developing criteria to continue therapy beyond RECIST progression. Cancer. 2013; 119:3761–8.18. Nishino M, Cardarella S, Dahlberg SE, Jackman DM, Ramaiya NH, Hatabu H, et al. Radiographic assessment and therapeutic decisions at RECIST progression in EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors. Lung Cancer. 2013; 79:283–8.
Article19. Asami K, Okuma T, Hirashima T, Kawahara M, Atagi S, Kawaguchi T, et al. Continued treatment with gefitinib beyond progressive disease benefits patients with activating EGFR mutations. Lung Cancer. 2013; 79:276–82.
Article20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article21. Li W, Ren S, Li J, Li A, Fan L, Li X, et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patients. Lung Cancer. 2014; 84:295–300.
Article22. Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011; 17:1169–80.
Article23. Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O'Connell A, Messineo MM, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014; 20:1698–705.
Article24. Wang Z, Chen R, Wang S, Zhong J, Wu M, Zhao J, et al. Quantification and dynamic monitoring of EGFR T790M in plasma cell-free DNA by digital PCR for prognosis of EGFR-TKI treatment in advanced NSCLC. PLoS One. 2014; 9:e110780.
Article25. Lecouvet FE, Talbot JN, Messiou C, Bourguet P, Liu Y, de Souza NM, et al. Monitoring the response of bone metastases to treatment with Magnetic Resonance Imaging and nuclear medicine techniques: a review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2014; 50:2519–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Small Cell Transformation in Pancreatic Metastasis from EGFR-Mutated Lung Adenocarcinoma Following TKI
- Overview of ALK and ROS1 Rearranged Lung Cancer
- Does the efficacy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor differ according to the type of EGFR mutation in non-small cell lung cancer?
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- Durable response to first-line treatment with AZD3759 (zorifertinib) in a patient with epithelial growth factor receptor mutated non-small cell lung cancer and untreated multiple brain metastasis