Cancer Res Treat.
2016 Jul;48(3):907-916. 10.4143/crt.2015.359.
The Effect of Induction Chemotherapy Using Docetaxel, Cisplatin, and Fluorouracil on Survival in Locally Advanced Head and Neck Squamous Cell Carcinoma: A Meta-Analysis
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. bhumsuk@snu.ac.kr
- 2Medical Research Collaborating Center, Seoul National University Hospital, Seoul, Korea.
- 3Department of Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2344063
- DOI: http://doi.org/10.4143/crt.2015.359
Abstract
- PURPOSE
The purpose of this study was to compare the survival of patients with locally advanced head and neck squamous cell carcinoma (LA-HNSCC) undergoing concurrent chemoradiotherapy (CRT) alone with that of patients undergoing induction chemotherapy (IC) using docetaxel, cisplatin, and 5-fluorouracil (TPF) followed by CRT.
MATERIALS AND METHODS
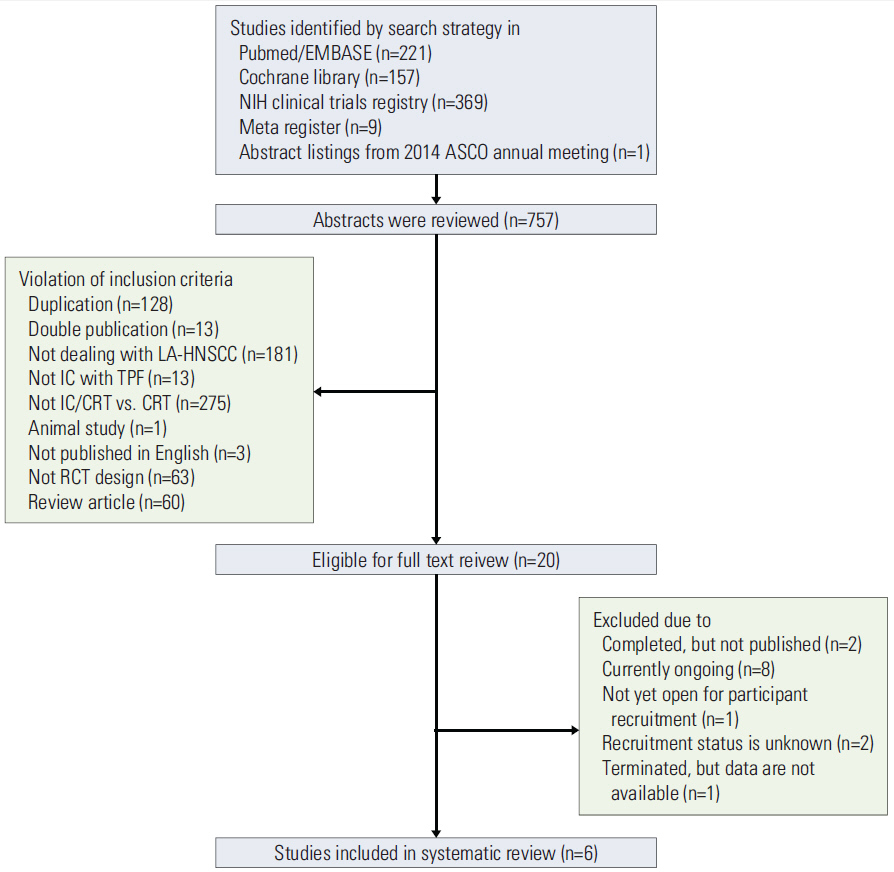
A search of the PubMed, EMBASE, and Cochrane Library databases was performed in April 2015 and abstracts from the American Society of Clinical Oncology meetings (2008-2014) were reviewed. Summaries of the results were pooled using a fixed-effect model, and the risk of bias was evaluated using the Cochrane tool.
RESULTS
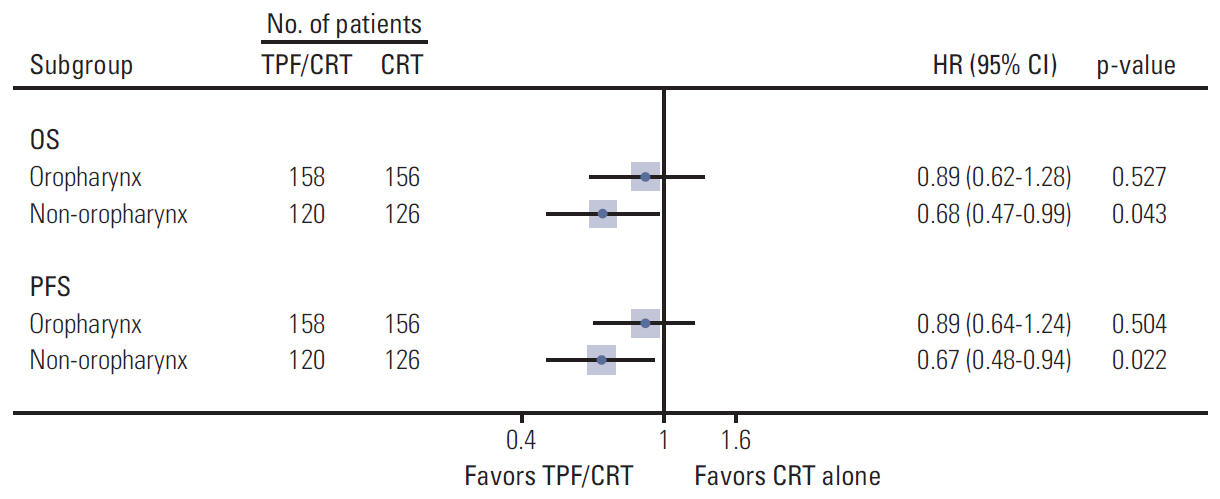
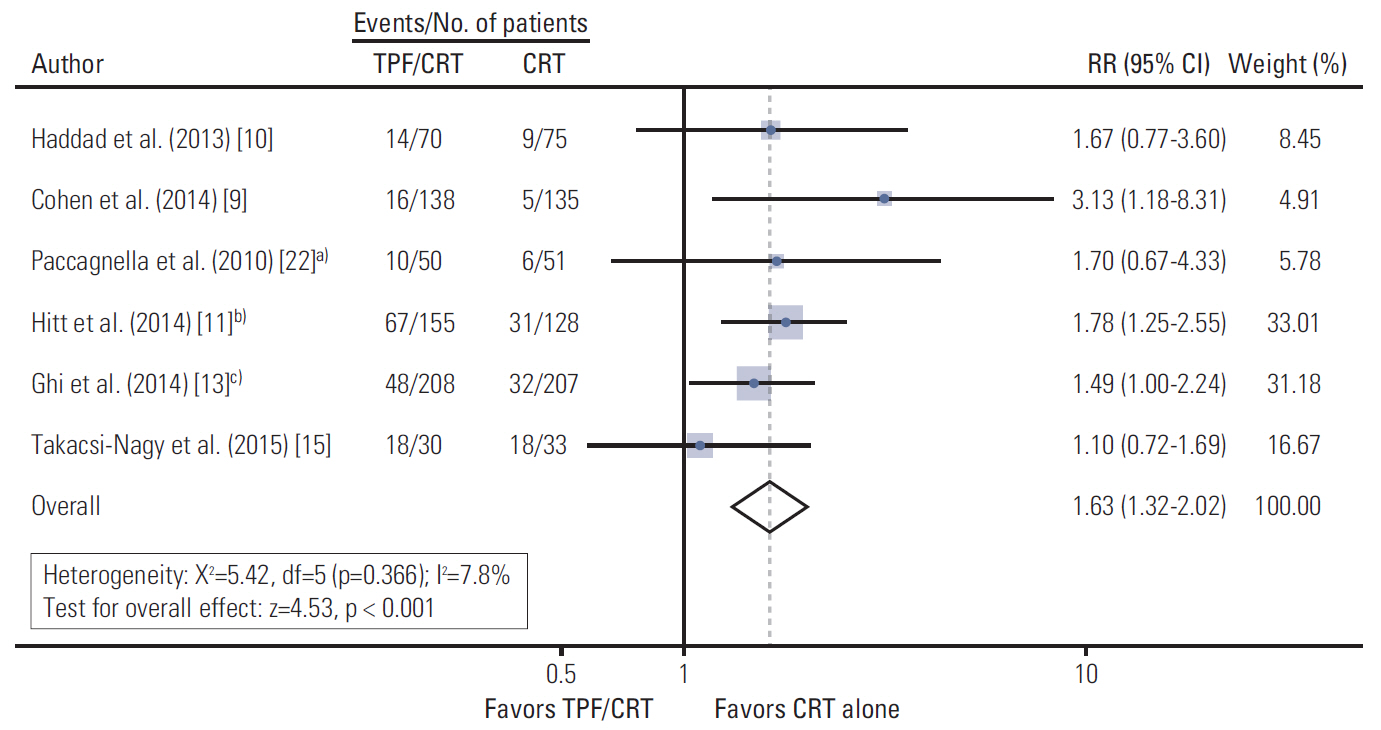
A total of six relevant trials comprising 1,280 patients were identified. There was no statistically significant overall survival (OS) advantage for TPF prior to CRT (TPF/CRT) over CRT alone (hazard ratio [HR] 0.92; 95% confidence interval [CI], 0.79 to 1.09; p=0.339). Progression-free survival (PFS) was significantly longer in the TPF/CRT arms (HR, 0.82; 95% CI, 0.70 to 0.95; p=0.009). Patients with non-oropharyngeal LA-HNSCC obtained the greatest OS and PFS benefits from TPF (HR, 0.68; 95% CI, 0.47 to 0.99; p=0.043 and HR, 0.67; 95% CI, 0.48 to 0.94; p=0.022, respectively). The complete response rate was significantly increased (risk ratio [RR], 1.34; 95% CI, 1.14 to 1.56; p < 0.001), and the distant metastasis rate tended to decrease (RR, 0.65; 95% CI, 0.40 to 1.04; p=0.071) in the TPF/CRT arms.
CONCLUSION
IC with TPF followed by CRT is not superior to CRT alone for OS. However, PFS and the complete response rate were significantly improved in the TPF/CRT arms. TPF/CRT for patients with nonoropharyngeal LA-HNSCC provided clear survival advantages.
MeSH Terms
Figure
Cited by 1 articles
-
Induction Chemotherapy as a Prognostication Index and Guidance for Treatment of Locally Advanced Head and Neck Squamous Cell Carcinoma: The Concept of Chemo-Selection (KCSG HN13-01)
Yun-Gyoo Lee, Eun Joo Kang, Bhumsuk Keam, Jin-Hyuk Choi, Jin-Soo Kim, Keon Uk Park, Kyoung Eun Lee, Hyo Jung Kim, Keun-Wook Lee, Min Kyoung Kim, Hee Kyung Ahn, Seong Hoon Shin, Hye Ryun Kim, Sung-Bae Kim, Hwan Jung Yun
Cancer Res Treat. 2022;54(1):109-117. doi: 10.4143/crt.2020.1329.
Reference
-
References
1. Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000; 355:949–55.2. Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008; 359:1143–54.
Article3. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991; 324:1685–90.4. Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010; 46:765–81.
Article5. Hitt R, Lopez-Pousa A, Martinez-Trufero J, Escrig V, Carles J, Rizo A, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005; 23:8636–45.
Article6. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007; 357:1705–15.
Article7. Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007; 357:1695–704.
Article8. Blanchard P, Bourhis J, Lacas B, Posner MR, Vermorken JB, Hernandez JJ, et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013; 31:2854–60.
Article9. Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014; 32:2735–43.
Article10. Haddad R, O'Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013; 14:257–64.
Article11. Hitt R, Grau JJ, Lopez-Pousa A, Berrocal A, Garcia-Giron C, Irigoyen A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014; 25:216–25.12. Faivre S, Albert S, Raymond E. Induction chemotherapy challenges for head and neck cancer. Lancet Oncol. 2013; 14:188–9.
Article13. Ghi MG, Paccagnella A, Ferrari D, Foa P, Cossu Rocca M, Verri E, et al. Concomitant chemoradiation (CRT) or cetuximab/RT (CET/RT) versus induction docetaxel/cisplatin/5-fluorouracil (TPF) followed by CRT or CET/RT in patients with locally advanced squamous cell carcinoma of head and neck (LASCCHN). A randomized phase III factorial study (NCT01086826). J Clin Oncol. 2014; 32(5S):Abstr 6004.14. Zhang L, Jiang N, Shi Y, Li S, Wang P, Zhao Y. Induction chemotherapy with concurrent chemoradiotherapy versus concurrent chemoradiotherapy for locally advanced squamous cell carcinoma of head and neck: a meta-analysis. Sci Rep. 2015; 5:10798.
Article15. Takacsi-Nagy Z, Hitre E, Remenar E, Oberna F, Polgar C, Major T, et al. Docetaxel, cisplatin and 5-fluorouracil induction chemotherapy followed by chemoradiotherapy or chemoradiotherapy alone in stage III-IV unresectable head and neck cancer: results of a randomized phase II study. Strahlenther Onkol. 2015; 191:635–41.16. Higgins JP, Green S. Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. West Sussex: John Wiley & Sons;2008.17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–88.
Article18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.
Article19. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50:1088–101.
Article20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.
Article21. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–16.22. Paccagnella A, Ghi MG, Loreggian L, Buffoli A, Koussis H, Mione CA, et al. Concomitant chemoradiotherapy versus induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by concomitant chemoradiotherapy in locally advanced head and neck cancer: a phase II randomized study. Ann Oncol. 2010; 21:1515–22.
Article23. Das LC, Karrison TG, Witt ME, Muller C, Stenson K, Blair EA, et al. Comparison of outcomes of locoregionally advanced oropharyngeal and non-oropharyngeal squamous cell carcinoma over two decades. Ann Oncol. 2015; 26:198–205.
Article24. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010; 363:24–35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction chemotherapy in patients with locally-advanced head and neck squamous cell carcinoma: docetaxel and cisplatin
- The Efficacy of an Induction Chemotherapy Combination with Docetaxel, Cisplatin, and 5-FU Followed by Concurrent Chemoradiotherapy in Advanced Head and Neck Cancer
- Organ Preservation for the Management of Locally Advanced Head and Neck Cancer
- Neoadjuvant chemotherapy with 5-fluorouracial infusion and cisplatin for locally advanced, untreated squamous cell carcinoma of the head and neck
- Induction Chemotherapy of Docetaxel and Cisplatin for the Elderly Patients with Squamous Cell Carcinoma of the Head and Neck