J Korean Ophthalmol Soc.
2013 Dec;54(12):1939-1944.
A Case of Acute Endophthalmitis Following a Dexamethasone Intravitreal Implant
- Affiliations
-
- 1Department of Ophthalmology, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea. ophtha@naver.com
Abstract
- PURPOSE
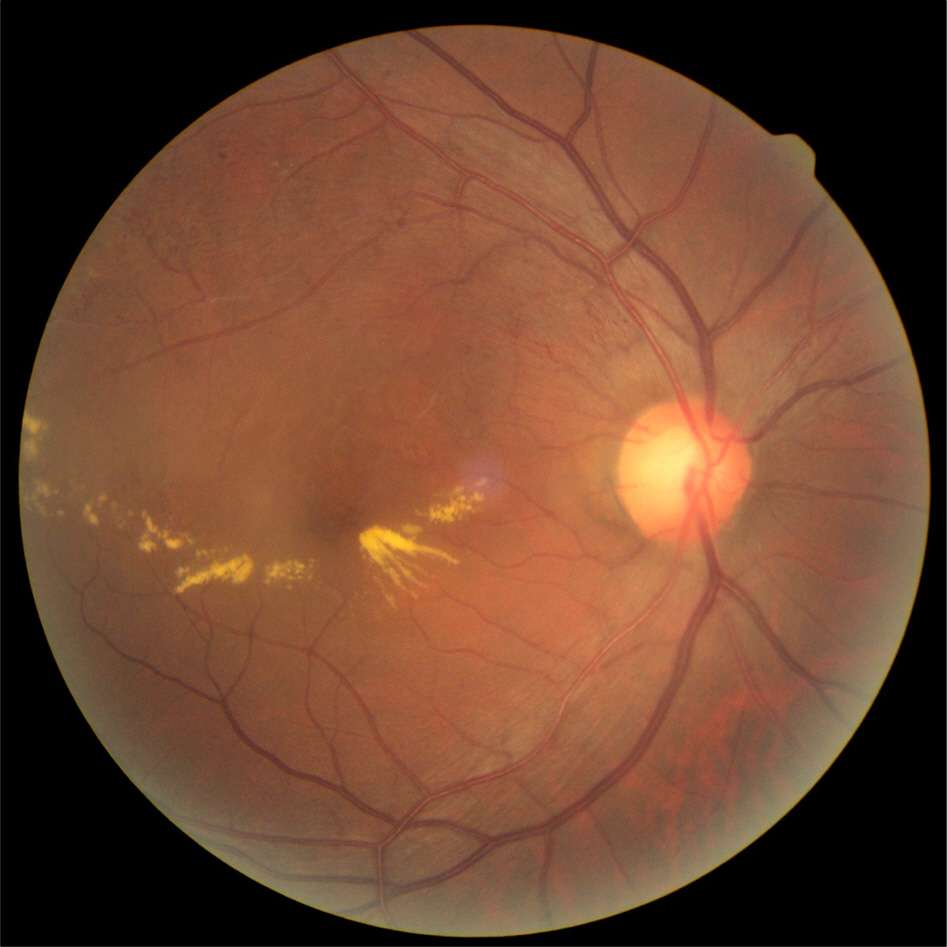
To report a case of acute endophthalmitis after a dexamethasone (Ozurdex(R)) intravitreal implant for macular edema (ME) secondary to branch retinal vein occlusion (BRVO).
CASE SUMMARY
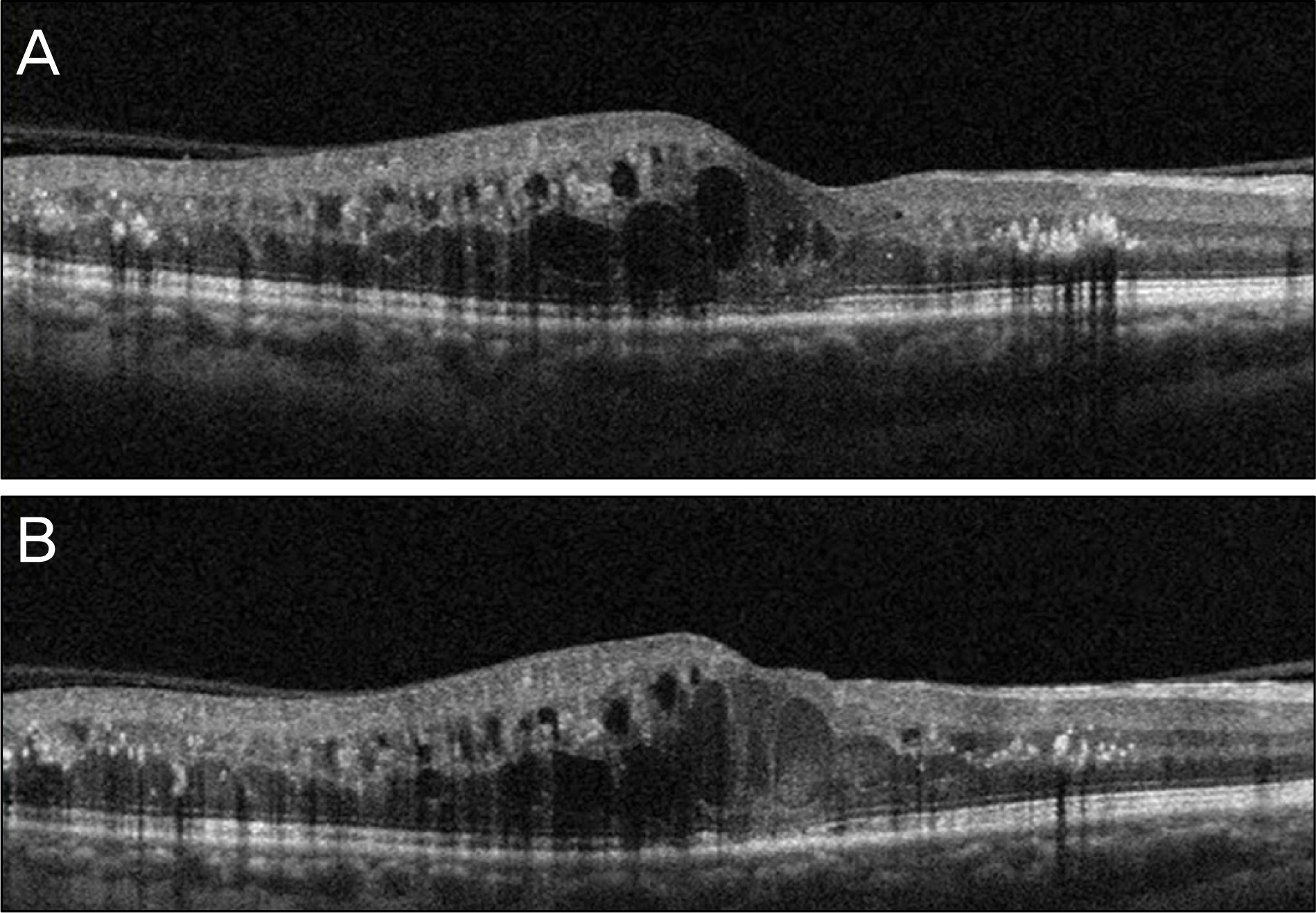
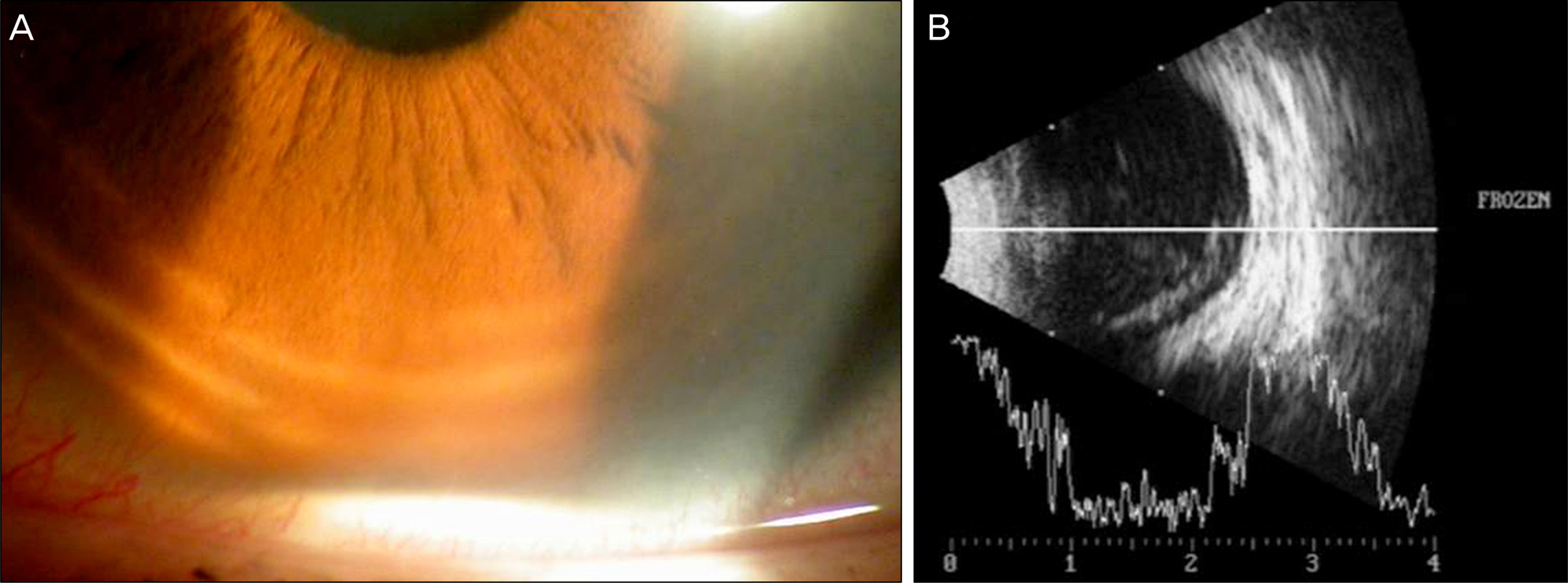
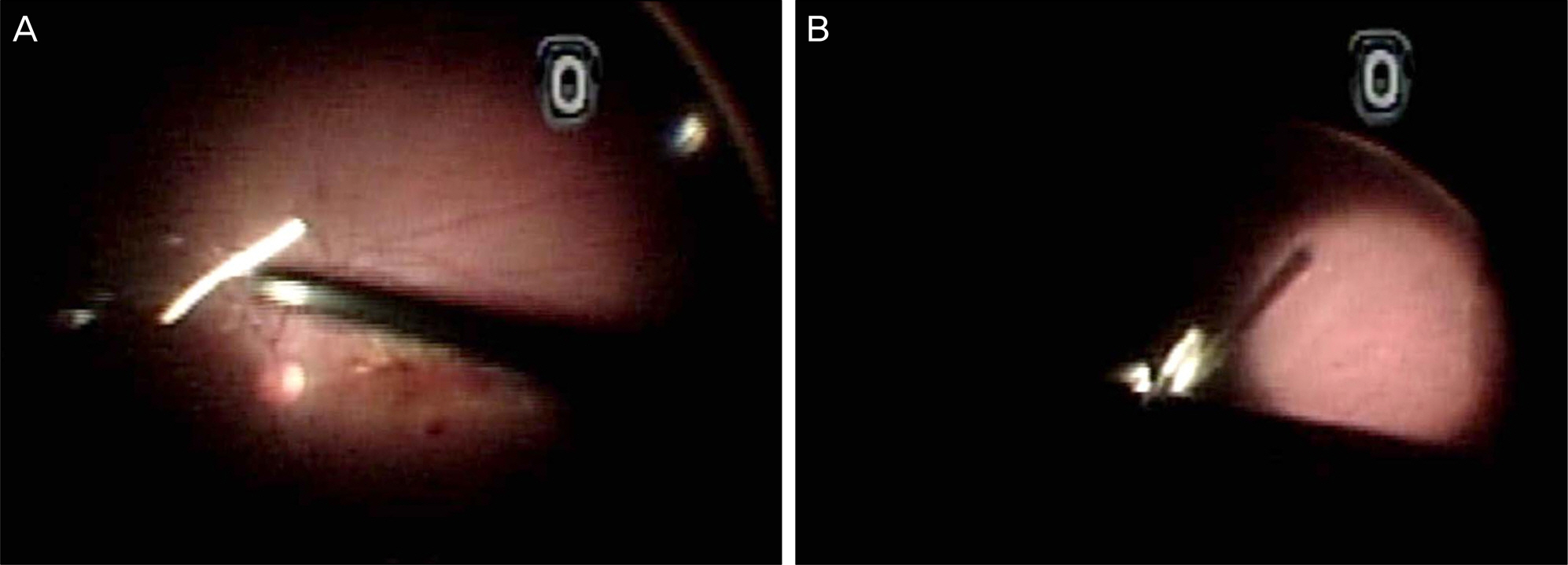
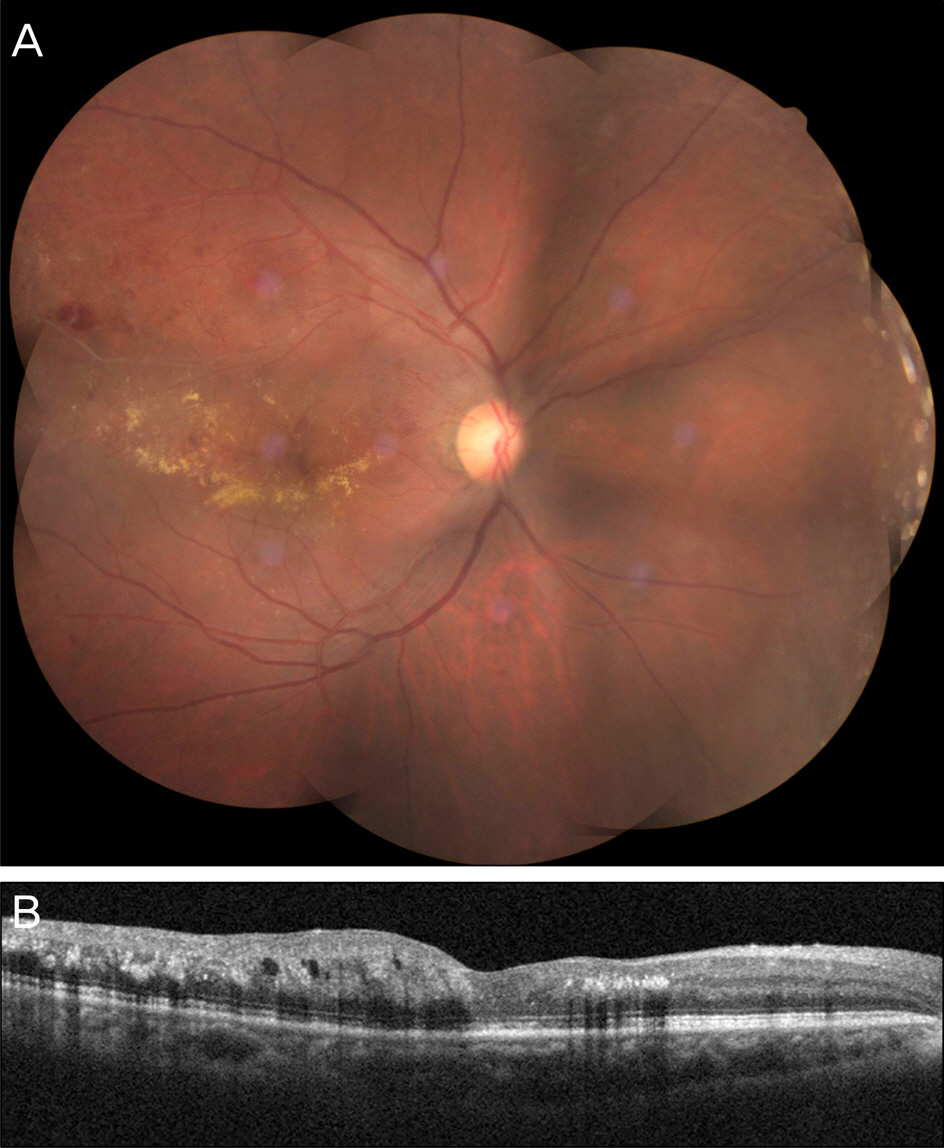
A 63-year-old male patient presented with decreased vision in the right eye due to ME secondary to BRVO. The patient was treated with an intravitreal bevacizumab injection, but ME did not improve. Two months after the injection, dexamethasone (Ozurdex(R)) intravitreal implantation was performed. Four days after the implantation, the patient visited our clinic complaining of severe visual disturbance. Slight conjunctival injection was observed and inflammatory cells and hypopyon were found in the anterior chamber. Fundus was not visible due to vitreous opacity. The patient was presumed to have acute endophthalmitis. Vitrectomy, intravitreal antibiotics injection, dexamethasone implant removal and phacoemulsification were performed. After treatment, the patient's fundus markedly improved, the inflammatory response was controlled and coagulase negative staphylococcus was detected from vitreous culture.
CONCLUSIONS
In cases of intravitreal dexamethasone implant associated with acute endophthalmitis, careful examination for diagnosis of endophthalmitis is recommended because the patient may not present with severe ocular pain and injection due to anti-inflammatory effect of corticosteroid.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jager RD, Aiello LP, Patel SC, Cunningham ET Jr.Risk of intra-vitreous injection: A comprehensive review. Retina. 2004; 24:676–98.2. Aiello LP, Brucker AJ, Chang S. . Evolving guidelines for in-travitreous injections. Retina. 2004; 24(5 Suppl):S3–19.
Article3. Sutter FK, Simpson JM, Gillies MC. Intravitreal triamcinolone for diabetic macular edema that persists after laser treatment: three-month efficacy and safety results of a prospective, randomized, double-masked, placebo-controlled clinical trial. Ophthalmology. 2004; 111:2044–9.4. Ip MS, Gottlieb JL, Kahana A. . Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004; 122:1131–6.5. Antcliff RJ, Spalton DJ, Stanford MR. . Intravitreal tri-amcinolone for uveitic cystoid macular edema: An optical coher-ence tomography study. Ophthalmology. 2001; 108:765–72.
Article6. Sutter FK, Gillies MC. Intravitreal triamcinolone for radiation in-duced macular edema. Arch Ophthalmol. 2003; 121:1491–3.7. Spaide RF, Sorenson J, Maranan L. Combined photodynamic ther-apy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2003; 110:1517–25.
Article8. Vasconcelos-Santos DV, Nehemy PG, Schachat AP, Nehemy MB. Secondary ocular hypertension after intravitreal injection of 4 mg of triamcinolone acetonide: incidence and risk factors. Retina. 2008; 28:573–80.9. Jung JW, Nam DH, Shyn KH. The complications after intravitreal injection of triamcinolone acetonide. J Korean Ophthalmol Soc. 2007; 48:55–62.10. Moshfeghi DM, Kaiser PK, Scott IU. . Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003; 136:791–6.
Article11. Gillies MC, Kuzniarz M, Craig J. . Intravitreal triamcinolone induced elevated intraocular pressure is associated with the devel-opment of posterior subcapsular cataract. Ophthalmology. 2005; 112:139–43.12. Rhee DJ, Peck RE, Belmont J. . Intraocular pressure alterations following intravitreal triamcinolone acetonide. Br J Ophthalmol. 2006; 90:999–1003.
Article13. Nelson ML, Tennant MT, Sivalingam A. . Infectious and presumed noninfectious endophthalmitis after intravitreal triamcinolone acetonide injection. Retina. 2003; 23:686–91.
Article14. Kuppermann BD, Blumenkranz MS, Haller JA. . Dexamethasone DDS Phase II Study Group. Randomized controlled study of an in-travitreous dexamethasone drug delivery system in patients with persistent macular edema. Arch Ophthalmol. 2007; 125:309–17.15. Haller JA, Bandello F, Belfort R Jr. . Randomized, sham-con-trolled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010; 117:1134–46.e3.
Article16. Chan A, Leung LS, Blumenkranz MS. Critical appraisal of the clinical utility of the dexamethasone intravitreal implant (Ozurdex) for the treatment of macular edema related to branch retinal vein occlusion or central retinal vein occlusion. Clin Ophthalmol. 2011; 5:1043–9.17. Pilli S, Kotsolis A, Spaide RF. . Endophthalmitis associated with intravitreal anti-vascular endothelial growth factor therapy in-jections in an office setting. Am J Ophthalmol. 2008; 145:879–82.
Article18. McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011; 31:654–61.19. Marchino T, Vela JI, Bassaganyas F. . Acuteonset endoph-thalmitis caused by alloiococcus otitidis following a dexamethasone intravitreal implant. Case Rep Ophthalmol. 2013; 4:37–41.20. Speaker MG, Milch FA, Shah MK. . Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991; 98:639–50.
Article21. Ta CN. Minimizing the risk of endophthalmitis following intra-vitreous injections. Retina. 2004; 24:699–705.
Article22. de Caro JJ, Ta CN, Ho HK. . Bacterial contamination of ocular surface and needles in patients undergoing intravitreal injections. Retina. 2008; 28:877–83.
Article23. Kreissig I, Degenring RF, Jones JB. [Intravitreal triamcinolone acetonide: complication of infectious and sterile endophthalmitis]. Ophthalmology. 2006; 103:30–4.24. Westfall AC, Osborn A, Kuhl D. . Acute endophthalmitis incidence. Arch Ophthalmol. 2005; 123:1075–7.
Article25. Bucher RS, Hall E, Reed DM. . Effect of intravitreal tri-amcinolone acetonide on susceptibility to experimental bacterial enodphthalmitis and subsequent response to treatment. Arch Ophthalmol. 2005; 123:649–53.26. Puliafito CA, Baker AS, Haaf J, Foster CS. Infectious endophthalmitis. Review of 36 cases. Ophthalmology. 1982; 89:921–9.27. Laatikainen L, Tarkkanen A. Early vitrectomy in the treatment of post-operative purulent endophthalmitis. Acta Ophthalmol (Copenh). 1987; 65:455–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Retinal Hemorrhage Following a Dexamethasone Intravitreal Implant
- Suspected Bacterial Endophthalmitis Following Sustained-release Dexamethasone Intravitreal Implant: A Case Report
- A Case of Repeated Intravitreal Dexamethasone Implantation for Treatment of Macular Edema after Scleral Fixation of Intraocular Lens
- Recurrence of Punctate Inner Choroidopathy with an Intravitreal Dexamethasone Implant
- Intraocular Pressure Changes in Patients with Retinal Vein Occlusion Treated with Intravitreal Dexamethasone Implant Insertion