J Korean Ophthalmol Soc.
2012 Jun;53(6):866-871.
A Case of Fungal Keratitis after Intracorneal Ring Segment Implantation for Keratoconus
- Affiliations
-
- 1Department of Ophthalmology, Dankook University Medical College, Cheonan, Korea. changmh@dankook.ac.kr
Abstract
- PURPOSE
To report a case of fungal keratitis 3 days after intracorneal ring segment (ICRS) implantation for keratoconus.
CASE SUMMARY
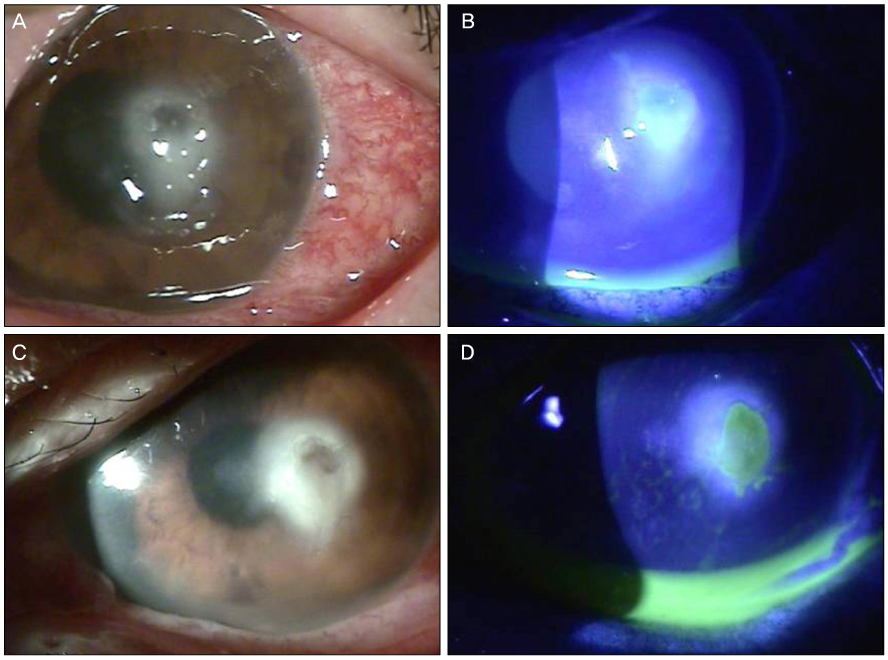
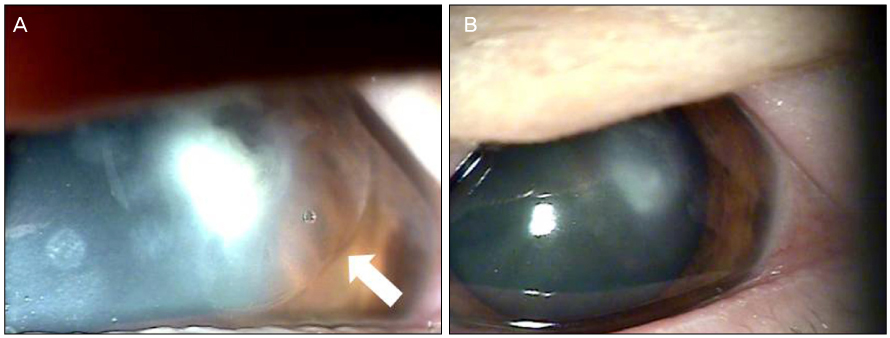
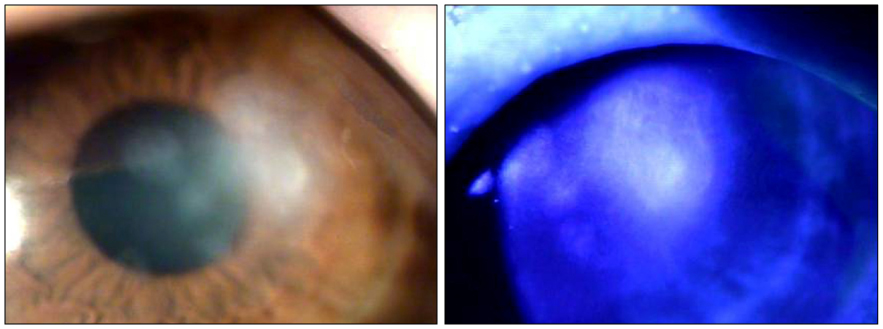
A 65-year-old woman was referred to our clinic with refractory infectious keratitis in her left eye 3 days after ICRS insertion for keratoconus. Slit lamp examinations revealed infiltrates around the incision site with cellular reaction in the anterior chamber after the ICRS had been removed. Corneal scrapings were obtained for staining and cultures, and intensive topical antibiotics were administered. Initial microscopy and cultures were negative. Despite the use of intensive topical antibiotics, there was no improvement. Hyphae were isolated from additional corneal scrapings. The patient's symptoms and corneal findings improved following administration of topical amphotericin B and oral itraconazole.
CONCLUSIONS
Infectious keratitis after ICRS implantation is an uncommon but sight-threatening complication. Fungal keratitis should also be considered if infectious keratitis after ICRS is unresponsive to antibiotics.
Keyword
MeSH Terms
Figure
Reference
-
1. Siganos CS, Kymionis GD, Kartakis N, et al. Management of keratoconus with Intacs. Am J Ophthalmol. 2003. 135:64–70.2. Boxer Wachler BS, Christie JP, Chandra NS, et al. Intacs for keratoconus. Ophthalmology. 2003. 110:1031–1040.3. Lovisolo CF, Fleming JF. Intracorneal ring segments for iatrogenic keratectasia after laser in situ keratomileusis or photorefractive keratectomy. J Refract Surg. 2002. 18:535–541.4. Kymionis GD, Siganos CS, Kounis G, et al. Management of post-LASIK corneal ectasia with Intacs inserts: one-year results. Arch Ophthalmol. 2003. 121:322–326.5. Siganos CS, Kymionis GD, Astyrakakis N, Pallikaris IG. Management of corneal ectasia after laser in situ keratomileusis with INTACS. J Refract Surg. 2002. 18:43–46.6. Hashemi H, Ghaffari R, Mohammadi M, et al. Microbial keratitis after INTACS implantation with loose suture. J Refract Surg. 2008. 24:551–552.7. Coskunseven E, Kymionis GD, Tsiklis NS, et al. One-year results of intrastromal corneal ring segment implantation (KeraRing) using femtosecond laser in patients with keratoconus. Am J Ophthalmol. 2008. 145:775–779.8. Shabayek MH, Alió JL. Intrastromal corneal ring segment implantation by femtosecond laser for keratoconus correction. Ophthalmology. 2007. 114:1643–1652.9. Schanzlin DJ, Abbott RL, Asbell PA, et al. Two-year outcomes of intrastromal corneal ring segments for the correction of myopia. Ophthalmology. 2001. 108:1688–1694.10. Ruckhofer J, Stoiber J, Alzner E, Grabner G. Multicenter European Corneal Correction Assessment Study Group. One year results of European Multicenter Study of intrastromal corneal ring segments. Part 2: complications, visual symptoms, and patient satisfaction. J Cataract Refract Surg. 2001. 27:287–296.11. Slade DS, Johnson JT, Tabin G. Acanthamoeba and fungal keratitis in a woman with a history of Intacs corneal implants. Eye Contact Lens. 2008. 34:185–187.12. Boorstein SM, Henk HJ, Elner VM. Atopy: a patient-specific risk factor for diffuse lamellar keratitis. Ophthalmology. 2003. 110:131–137.13. McAlister JC, Ardjomand N, Ilari L, et al. Keratitis after intracorneal ring segment insertion for keratoconus. J Cataract Refract Surg. 2006. 32:676–678.14. Ruckhofer J, Twa MD, Schanzlin DJ. Clinical characteristics of lamellar channel deposits after implantation of intacs. J Cataract Refract Surg. 2000. 26:1473–1479.15. Bourcier T, Borderie V, Laroche L. Late bacterial keratitis after implantation of intrastromal corneal ring segments. J Cataract Refract Surg. 2003. 29:407–409.16. Hofling-Lima AL, Branco BC, Romano AC, et al. Corneal infections after implantation of intracorneal ring segments. Cornea. 2004. 23:547–549.17. Shehadeh-Masha'our R, Modi N, Barbara A, Garzozi HJ. Keratitis after implantation of intrastromal corneal ring segments. J Cataract Refract Surg. 2004. 30:1802–1804.18. Zare MA, Hashemi H, Salari MR. Intracorneal ring segment implantation for the management of keratoconus: safety and efficacy. J Cataract Refract Surg. 2007. 33:1886–1891.19. Coskunseven E, Kymionis GD, Tsiklis NS, et al. Complications of intrastromal corneal ring segment implantation using a femtosecond laser for channel creation: a survey of 850 eyes with keratoconus. Acta Ophthalmol. 2011. 89:54–57.20. Kwitko S, Severo NS. Ferrara intracorneal ring segments for keratoconus. J Cataract Refract Surg. 2004. 30:812–820.21. Galvis V, Alejandro T, Julián D, et al. Late bacterial keratitis after intracorneal ring segments (Ferrara ring) insertion for keratoconus. Cornea. 2007. 26:1282–1284.22. Chalasani R, Beltz J, Jhanji V, Vaipayee RB. Microbial keratitis following intracorneal ring segment implantation. Br J Ophthalmol. 2010. 94:1541.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracorneal Ring Segment Implantation for the Management of Keratoconus: Short-Term Safety and Efficacy
- Clinical Results of Intacs(R) Ring Implantation in Keratoconus or Keratectasia
- The Clinical Results of Intacs(R) Ring Implantation by Manual Tunnel Creation in Patients with Keratoconus
- Effect of Sequential Intrastromal Corneal Ring Segment Implantation and Corneal Collagen Crosslinking in Corneal Ectasia
- Sequential Intrastromal Corneal Ring Implantation and Cataract Surgery in a Severe Keratoconus Patient with Cataract