J Korean Ophthalmol Soc.
2008 Nov;49(11):1723-1728.
Influence of Surface Fluid during Photorefractive Keratectomy Using a 213-nm Solid-State Laser
- Affiliations
-
- 1Department of Ophthalmology, Sungkyunkwan University School of Medicine, Kangbuk Samsung Hospital, Seoul, Korea. sashimi0@naver.com
- 2Department of Ophthalmology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
Abstract
- PURPOSE
To investigate the effect of surface fluid on the ablation rate and efficacy of 213-nm solid-state laser during photorefractive keratectomy (PRK). METHODS: Twelve rabbits (24 eyes) underwent myopic PRK for the correction of 10 diopters using 213-nm solid-state laser. Photoablation was performed with removal of corneal surface fluid using the Weckcel(R) sponge every 5 seconds in one eye and without removal of corneal surface fluid in the control eye. The mean central corneal thickness (CCT) was evaluated preoperatively, and at 1 week, 4 weeks postoperatively.
RESULTS
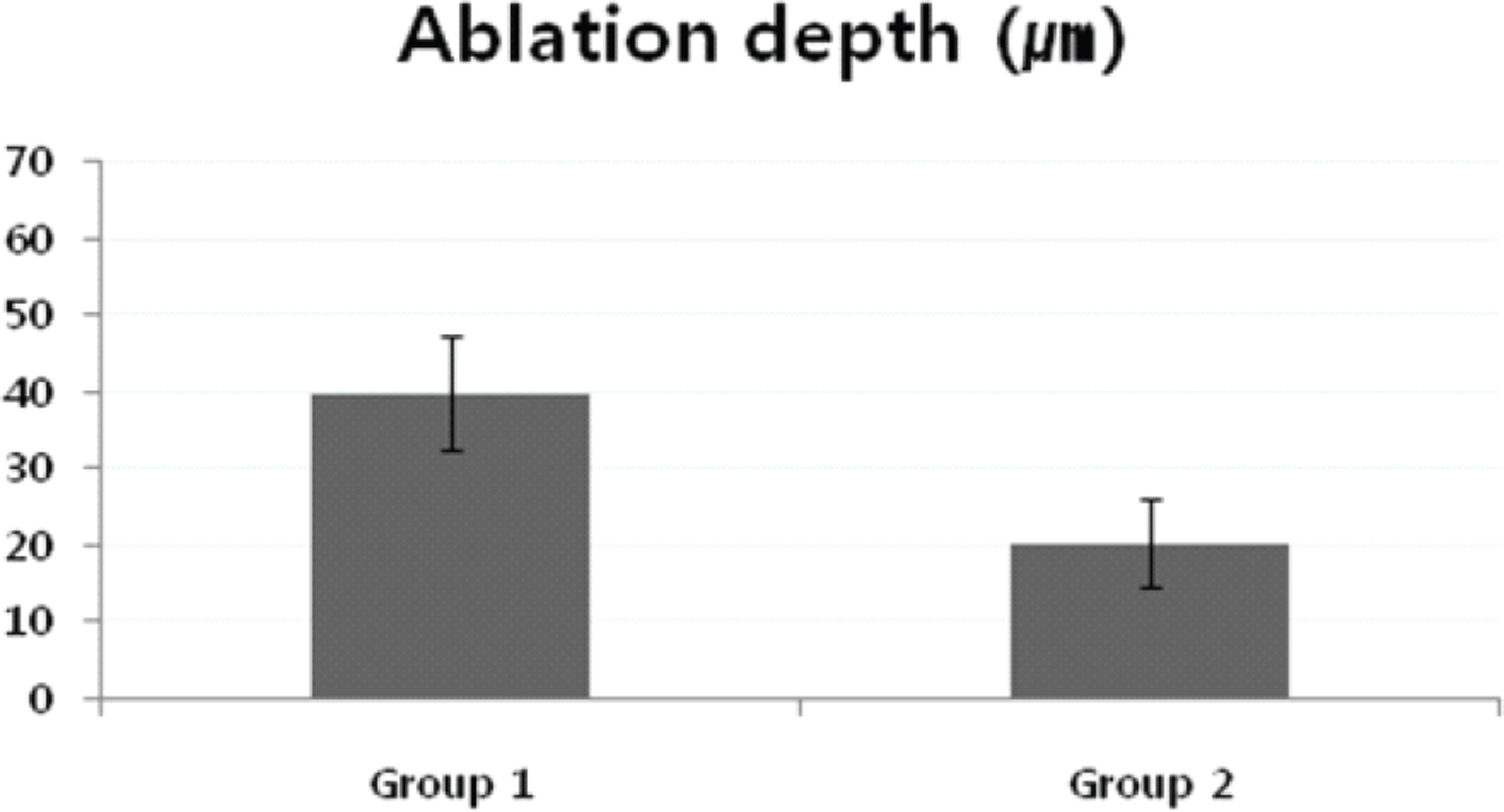
The mean CCT of group 1 (with removal of corneal surface fluid) were 361.3+/-13.9 micrometer preoperatively and 321.4+/-18.5 micrometer at 4 weeks postoperatively. The mean CCT of group 2 (without removal of surface fluid) were 358.7+/-8.9 micrometer preoperatively and 338.4+/-12.0 micrometer at 4 weeks postoperatively. The mean ablation depths were 39.8+/-7.4 micrometer in group 1 and 20.3+/-5.8 micrometer in group 2 at 4 weeks postoperatively p<0.05).
CONCLUSIONS
Induced corneal surface fluid during PRK may influence the ablation efficacy and accuracy of solid-state laser. This result should be considered in clinical trialswith 213-nm solid-state laser, especially in high myopes.
Keyword
Figure
Reference
-
References
1. Ediger MN, Pettit GH, Matchette LS. In vitro measurements of cytotoxic effects of 193nm and 213nm laser pulses at subablative fluences. Lasers Surg Med. 1997; 21:88–93.2. Dair GT, Pelouch WS, Van Saarloos PP, et al. Investigation of corneal ablation efficiency using ultraviolet 213-nm solid state laser pulses. Invest Ophthalmol Vis Sci. 1999; 40:2752–6.3. Ren Q, Simon G, Parel JM. Ultraviolet solid-state laser (213-nm) photorefractive keratectomy: in vitro study. Ophthalmology. 1993; 100:1828–34.4. Ren Q, Simon G, Legeais JM, et al. Ultraviolet solid-state laser (213-nm) photorefractive keratectomy: in vivo study. Ophthalmology. 1994; 101:883–9.5. Tsiklis NS, Kymionis GD, Kounis GA, et al. Photorefractive keratectomy using solid state laser 213nm and excimer laser 193nm: A randomized, contralateral, comparative, experimental study. Invest Ophthalmol Vis Sci. 2008; 49:1415–20.6. Dougherty PJ, Wellish KL, Maloney RK. Excimer laser ablation rate and corneal hydration. Am J Ophthalmol. 1994; 118:169–76.
Article7. Fields CR, Taylor SM, Barker FM. Effect of corneal edema upon the smoothness of excimer laser ablation. Optom Vis Sci. 1994; 71:109–14.
Article8. Campos M, Wang XW, Hertzog L, et al. Ablation rates and surface ultrastructure of 193nm excimer laser keratectomies. Invest Ophthalmol Vis Sci. 1993; 34:2493–500.9. Dair GT, Ashman RA, Eikelboom RH, et al. Absorption 193- and 213-nm laser wavelengths in sodium chloride solution and balancedsalt solution. Arch Ophthalmol. 2001; 119:533–7.10. Lembares A, Hu X-H, Kalmus GW. Absorption spectra of corneas in the far ultraviolet region. Invest Ophthalmol Vis Sci. 1997; 38:1283–7.11. Ito T, Ito A, Hieda K, Kobayashi K. Wavelength dependence of inactivation and membrane damage to Saccharomyces cerevisiae cells by monochromatic synchrotron vacuum-UV radiation (145-190-nm). Radiat Res. 1983; 96:532–48.12. Hieda K, Ito T. Action spectra for inactivation and membrane damage of Saccharomyces cerevisiae cells irradiated in vacuum by monochromatic synchrotron UV radiation (155-250-nm). Photochem Photobiol. 1986; 44:409–11.13. Munakata N, Hieda K, Kobayashi K, et al. Action spectra in ultraviolet wavelengths (150-250-nm) for inactivation and mutagenesis of Bacillus subtilis spores obtained with synchrotron radiation. Photochem Photobiol. 1986; 44:385–90.14. Kornmehl EW, Steinert RF, Puliafito CA. A comparative study of masking fluids for excimer laser phototherapeutic keratectomy. Arch Ophthalmol. 1991; 109:860–3.
Article15. Förster W, Grewe S, Atzler U, et al. Phototherapeutic keratectomy in corneal diseases. Refract Corneal Surg. 1993; 9:S85–90.
Article16. Thompson V, Durrie DS, Cavanaugh TB. Philosophy and technique for excimer laser phototherapeutic keratectomy. Refract Corneal Surg. 1993; 9:S81–5.
Article17. Oshika T, Klyce SD, Smolek MK, McDonald MB. Corneal hydration and central islands after excimer laser photorefractive keratectomy. J Cataract Refract Surg. 1998; 24:1575–80.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Ocular Deviation after Excinier Laser Photorefractive Keratectomy
- Complications after Excimer Laser Photorefractive Keratectomy in Myopia
- Phototherapeutic Keratectomy for the Treatment of Persistent Epithelial Defect
- Comparison of Photorefractive Keratectomy and Laser Epithelial Keratomileusis for Low to Moderate Myopia
- Corneal Epithelial Wound Healing After Excimer Laser Photorefractive Keratectomy