J Korean Soc Endocrinol.
2005 Apr;20(2):134-141.
Meanings of Expression of Vascular Endothelial Growth Factor in Thyroid Tumors
- Affiliations
-
- 1Department of Internal Medicine, Inje University College of Medicine, Korea.
- 2Department of Internal Medicine, Kosin University College of Medicine, Korea.
- 3Department of Pathology, Kosin University College of Medicine, Korea.
Abstract
-
BACKGROUND: Angiogenesis is essential for tumor growth and metastasis. Vascular endothelial growth factor(VEGF), also known as vascular permeability factor(VPF), is an angiogenic factor that plays important roles in tumor growth. Angiogenesis studies on VEGF deal with various types of malignant tumors, but little is known about the role or significance of VEGF in human thyroid neoplasms. Therefore, this study was performed to determine whether the VEGF expression in different histological types of thyroid tumors is altered and to see if there was a relationship between the expression of VEGF and either metastasis or the invasiveness of thyroid carcinomas.
METHODS
Forty-two cases that underwent thyroidectomy at Kosin Medical Center, between March, 1999 and February, 2000, were included in this study. Of the 42 cases, 27 were malignant(26 papillary carcinoma, 1 Hurthle cell carcinoma) and 15 were benign lesions. The expression of VEGF was determined by immunohistochemistry using paraffin embedded thyroid tissue blocks, and was quantified as negative(absent), +(1~24%), ++(25~49%), +++(50~74%) and ++++(> or =75%), according to the extent of positive cells.
RESULTS
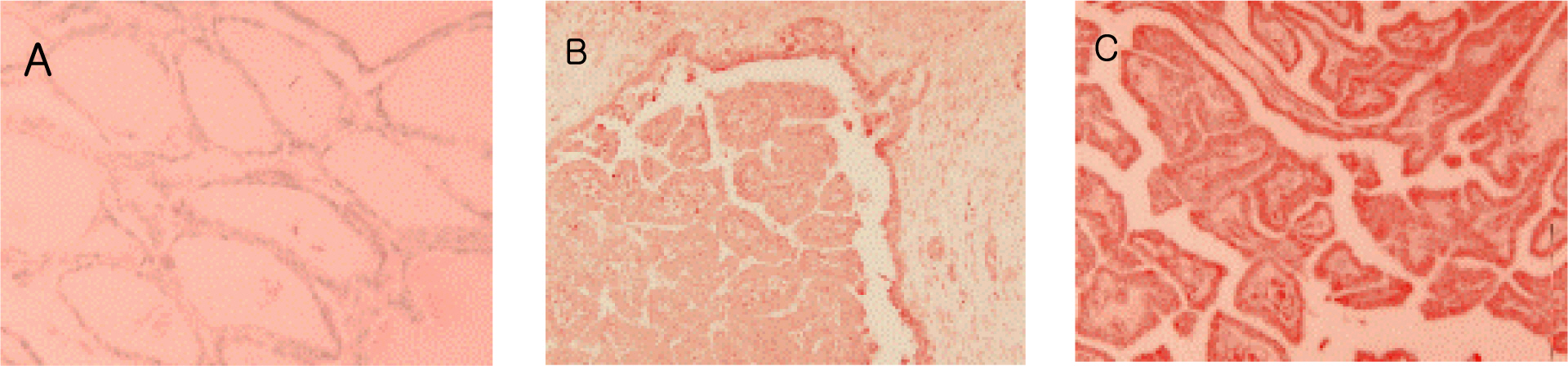
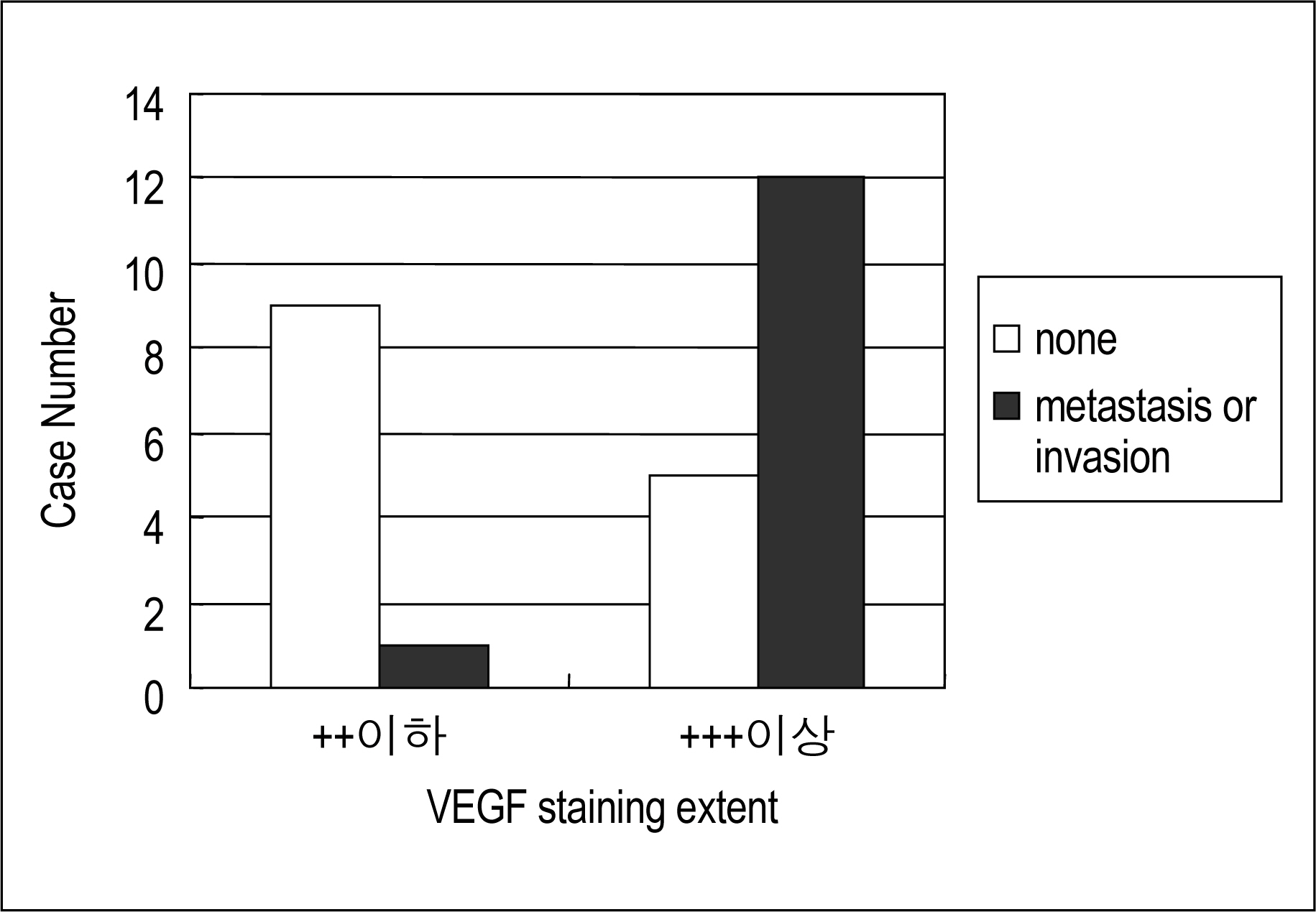
VEGF was stained with red-brown colored granules in the cytoplasm of the thyroid tumor epithelium and was expressed in 27 of the 42 cases(+1, ++8, +++5, ++++13). Most malignant tumors(24 of 27 cases) were stained with VEGF, but only 3 of the 15 benign tumors cases were stained(P<0.001). When the VEGF expression was divided into ++ or below and +++ or above groups, the expression of VEGF was much more extensive in the malignant than benign tumors(P<0.001). Of the 27 malignant tumors cases, lymph node metastasis and/or invasion was noted in 13. VEGF expression was more extensive in malignant tumors with lymph node metastasis and/or invasion than in those without(P<0.001).
CONCLUSION
In this study, the rate and extent of VEGF expression were greater in the malignant than the benign thyroid tumors, and also the extent of VEGF expression was the extent of VEGF greater in the malignant tumors with lymph node metastasis and/or invasion than those without
MeSH Terms
-
Angiogenesis Inducing Agents
Capillary Permeability
Carcinoma, Papillary
Cytoplasm
Epithelium
Humans
Immunohistochemistry
Lymph Nodes
Neoplasm Metastasis
Paraffin
Thyroid Gland*
Thyroid Neoplasms
Thyroidectomy
Vascular Endothelial Growth Factor A*
Angiogenesis Inducing Agents
Paraffin
Vascular Endothelial Growth Factor A
Figure
Reference
-
References
1. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990; 82:4–6.
Article2. Carmeliet P, Jain RK. Angiogenesis in cancer and other disease. Nature. 2000; 407:249–257.3. Turner HE, Harris AL, Melmed S, Wass JA. Angiogenesis in endocrine tumors. Endocr Rev. 2003; 24:600–632.
Article4. Moscatelli DA, Gross J, Rifkin D. Angiogenesis factors stimulate plasminogen activator and collagenase production by capillary endothelial cells. J Pathol. 1993; 170(Suppl):338a.5. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J Med. 1991; 324:1–8.6. Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991; 181:902–906.
Article7. Mandriota SJ, Seghezzi G, Vassalli JD, Ferrara N, Wasi S, Mazzieri R, Mignatti P, Pepper MS. Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J Biol Chem. 1995; 270:9709–9716.
Article8. Semenza GL. Angiogenesis in ischemic and neoplastic disordes. Annu Rev Med. 2003; 54:17–28.9. Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992; 3(2):65–71.10. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246:1306–1309.
Article11. Tischer E, Mitchell R, Hartman T, Silva M, Gos-podarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991; 266:11947–11954.
Article12. Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991; 5:1806–1814.
Article13. Frank S, Hubner G, Breier G, Longaker MT, Green-halgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995; 270:12607–12613.14. Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994; 265:1582–1584.
Article15. Kieser A, Weich HA, Brandner G, Marme D, Kolch W. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene. 1994; 9:963–969.16. Kolch W, Martiny-Baron G, Kieser A, Marme D. Regulation of the expression of the VEGF/ VPS and its receptors: role in tumor angiogenesis. Breast Cancer Res Treat. 1995; 36:139–155.17. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995; 146:1029–1039.18. Senger DR, Van de Water L, Brown LF, Nagy JA, Yeo KT, Yeo TK, Berse B, Jackman RW, Dvorak AM, Dvorak HF. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993; 12:303–324.
Article19. Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, Dvorak HF. Expression of vascular permeability factor(vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993; 53:4727–4735.20. Viglietto G, Maglione D, Rambaldi M, Cerutti J, Romano A, Trapasso F, Fedele M, Ippolito P, Chiappetta G, Botti G, et al. Upregulation of vascular endothelial growth factor (VEGF) and down-regulation of placenta growth factor (PlGF) associated with malignancy in human thyroid tumors and cell lines. Oncogene. 1995; 11:1569–1579.21. Katoh R, Miyagi E, Kawaoi A, Hemmi A, Ko-miyama A, Oyama T, Shibuya M. Expression of vascular endothelial growth factor (VEGF) in human thyroid neoplasms. Hum Pathol. 1999; 30:891–897.
Article22. Hedinger C, Williams ED, Sobin LH. Histological typing of thyroid tumors WHO 2nd Ed. Berlin: Springer Verlag;1998.23. Hanahan D, Folkman J. Patterns and emerging mechanism of angiogenic switch during tumorigenesis. Cell. 1996; 86:353–364.24. Staibano S, Boscaino A, Salvatore G, Orabona P, Palombini L, De Rosa G. The prognostic significance of tumor angiogenesis in nonaggressive and aggressive basal cell carcinoma of the human skin. Hum Pathol. 1996; 27:695–700.
Article25. Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992; 84:1875–1887.
Article26. Bremer GL, Tiebosch AT, van der Putten HW, Schouten HJ, de Haan J, Arends JW. Tumor angiogenesis: an independent prognostic parameter in cervical cancer. Am J Obstet Gynecol. 1996; 174:126–131.
Article27. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25.
Article28. Wong MP, Cheung N, Yuen ST, Leung SY, Chung LP. Vascular endothelial growth factor is up-regulated in the early pre-malignant stage of colorectal tumour progression. Int J Cancer. 1999; 81:845–850.
Article29. Toi M, Hoshina S, Takayanagi T, Tominaga T. Association of vascular endothelial growth factor expression with tumor angiogenesis and with early relapse in primary breast cancer. Jpn J Cancer Res. 1994; 85:1045–1049.
Article30. Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996; 77:858–863.
Article31. Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995; 55:3964–3968.32. Nagura S, Katoh R, Miyagi E, Shibuya M, Kawaoi A. Expression of vascular endothelial growth factor (VEGF) and VEGF receptor-1 (Flt-1) in Graves disease possibly correlated with increased vascular density. Human path. 2001; 32:10–17.
Article33. Klein M, Picard E, Vignaud JM, marie B, Bresler L, Toussaint B, Weryha G, Duprez A, Leclere J. Vascular endothelial growth factor gene and protein: strong expression in thyroiditis and thyroid carcinoma. J Endocrinol. 1999; 161:41–49.
Article34. Soh EY, Duh QY, Sobhi SA, Young DM, Epstein HD, Wong MG, Garcia YK, Min YD, Grossman RF, Siperstein AE, Clark OH. Vascular Endothelial Growth Factor Expression Is Higher in Differentiated Thyroid Cancer than in Normal or Benign Thyroid. J Clin Endocrinol Metab. 1997; 82:3741–3747.
Article35. Bunone G, Vigneri P, Mariani L, Buto S, Collini P, Pilotti S, Pierotti MA, Bongarzone I. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol. 1999; 155:1967–1976.
Article36. Fenton C, Patel A, Dinauer C, Robie DK, Tuttle RM, Francis GL. The expression of vascular endothelial growth factor and the type 1 vascular endothelial growth factor receptor correlate with the size of papillary thyroid carcinoma in children and young adults. Thyroid. 2000; 10:349–357.
Article37. Jubb AM, Pham TQ, Hanby AM, Frantz GD, Peale FV, Wu TD, Koeppen HW, Hillan KJ. Expression of vascular endothelial growth factor, hypoxia inducible factor 1alpha, and carbonic anhydrase IX in human tumours. J Clin Pathol. 2004; 57:504–512.38. Lennard CM, Patel A, Wilson J, Reinhardt B, Tuman C, Fenton C, Blair E, Francis GL, Tuttle RM. Intensity of vascular endothelial growth factor expression is associated with increased risk of recurrence and decreased disease-free survival in papillary thyroid cancer. Surgery. 2001; 129:552–558.
Article39. Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, Leclère J, Duprez A, Weryha G. Increased Expression of the Vascular Endothelial Growth Factor Is a Pejorative Prognosis Marker in Papillary Thyroid Carcinoma. J Clin Endocrinol Metab. 2001; 86:656–658.
Article40. Kilicarslan AB, Ogus M, Arici C, Pestereli HE, Cakir M, Karpuzoglu G. Clinical importance of vascular endothelial growth factor (VEGF) for papillary thyroid carcinomas. APMIS. 2003; 111:439–443.
Article41. Huang SM, Lee JC, Wu TJ, Chow NH. Clinical Relevance of Vascular Endothelial Growth Factor for Thyroid Neoplasms. World J surg. 2001; 25:302–306.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Vascular Endothelial Growth Factor (VEGF) in Papillary Thyroid Carcinoma
- Expression of Vascular Endothelial Growth Factor in Astrocytic Tumors: Correlation to Peritumoral Brain Edema and Microvasculature
- Expression of Vascular Endothelial Growth Factor : Clinical Implications in Cervical Neoplasia
- Expression of Vascular Endothelial Growth Factor and Peritumoral Brain Edema in Intracranial Meningiomas
- Vascular Endothelial Growth Factor Expression in Human Trohoblast Cell Line