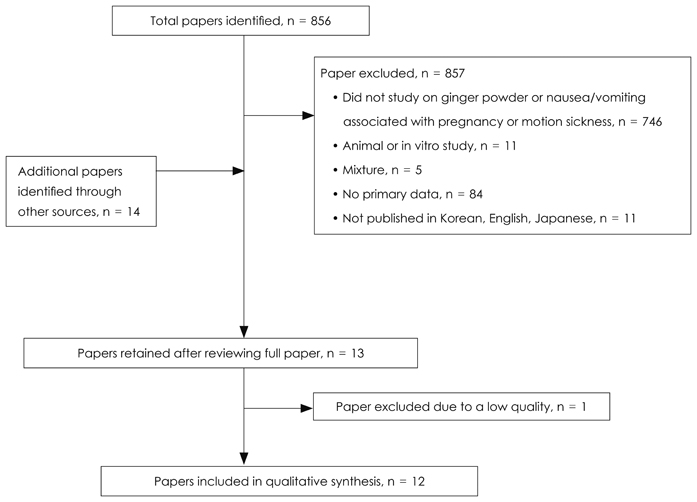
J Nutr Health.
2014 Feb;47(1):45-50.
Systematic review of the effect of dried ginger powder on improvement of nausea and vomiting associated with early pregnancy or motion sickness
- Affiliations
-
- 1Biofood CRO Co., Ltd., Seoul 120-160, Korea.
- 2Biofood Network Center, Ewha Womans University, Seoul 120-750, Korea. orank@ewha.ac.kr
- 3Department of Food Science and Technology, Seoul National University of Science and Technology, Seoul 139-743, Korea.
- 4Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 120-750, Korea.
Abstract
- PURPOSE
Ginger (Zingiber officinale) has been widely used as an antiemetic agent. This systematic review was aimed at evaluation of the effect of dried ginger powder supplementation on improvement of nausea and vomiting associated with early pregnancy or motion sickness.
METHODS
We searched Pubmed, Cochrane, Science Direct, and KISS (Korean studies Information Service System) using keywords such as ginger or Zingiber officinale in combination with nausea, vomiting, motion sickness, or pregnancy, published in March 2013.
RESULTS
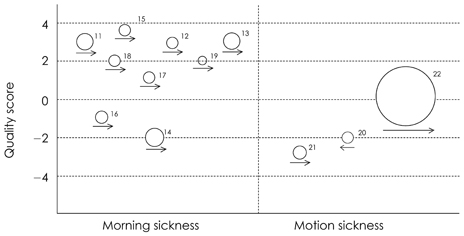
The strength of the evidence was evaluated on the selected 12 RCTs (randomized controlled trials). Eleven trials including 2,630 subjects showed that supplementation with dried ginger powder resulted in significant improvement of nausea or vomiting related to early pregnancy or motion sickness. Among the nine studies including 809 women in early pregnancy before 20 weeks of gestation, ginger supplementation was superior to placebo in five studies (n = 305), and as effective as positive control (vitamin B6 or dimenhydrinate) in four studies (n = 504). Ginger intake significantly reduced the episodes or severity of vomiting related to motion sickness compared to placebo or showed the same effect as several antiemetic drugs in two studies (n = 1,821).
CONCLUSION
Our findings added evidence indicating that ginger powder supplements might improve the symptoms of nausea or vomiting related to early pregnancy or motion sickness without significant adverse events.
MeSH Terms
Figure
Reference
-
1. Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005; 12(9):684–701.
Article2. National Institutes of Health (US). Dietary Supplement Label Database (DSLD) [Internet]. Bethesda (MA): National Institutes of Health;2013. 09. cited 2013 Sep 18. Available from: http://www.dsld.nlm.nih.gov/dsld/.3. Flake ZA, Scalley RD, Bailey AG. Practical selection of antiemetics. Am Fam Physician. 2004; 69(5):1169–1174.4. Yamahara J, Rong HQ, Iwamoto M, Kobayashi G, Matsuda H, Fujimura H. Active components of ginger exhibiting anti-serotonergic action. Phytother Res. 1989; 3(2):70–71.
Article5. Huang QR, Iwamoto M, Aoki S, Tanaka N, Tajima K, Yamahara J, Takaishi Y, Yoshida M, Tomimatsu T, Tamai Y. Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem Pharm Bull (Tokyo). 1991; 39(2):397–399.
Article6. Korea Food & Drug Administration. Guidance for the Evaluation on the Functionality of Health Functional Food. Seoul: Korea Food & Drug Administration;2008. p. 30–32.7. U.S. Food and Drug Administration. Guidance for industry: evidence-based review system for the scientific evaluation of health claims [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;2009. 01. cited 2013 Nov 15. Available from: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm073332.htm.8. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (IT). Scientific and technical guidance for the preparation and presentation of an application for authorisation of a health claim (revision 1). EFSA J [serial on the Internet]. 2011. cited 2013 Nov 17. 9(5):2170. [about 36 p.] Available from: http://www.efsa.europa.eu/en/efsajournal/pub/2170.htm.9. Jeong S, Kim JY, Paek JE, Kim J, Kwak JS, Kwon O. Systematic review of the effect of omega-3 fatty acids on improvement of blood flow while focused on evaluation of claims for health functional food. J Nutr Health. 2013; 46(3):226–238.
Article10. Kim JY, Jeong S, Paek JE, Kim J, Kwak JS, Lee YJ, Kang TS, Kwon O. Systematic review of the effect of coenzyme Q10 on antioxidant capacity while focused on evaluation of claims for health functional food. J Nutr Health. 2013; 46(3):218–225.
Article11. Sripramote M, Lekhyananda N. A randomized comparison of ginger and vitamin B6 in the treatment of nausea and vomiting of pregnancy. J Med Assoc Thai. 2003; 86(9):846–853.12. Ensiyeh J, Sakineh MA. Comparing ginger and vitamin B6 for the treatment of nausea and vomiting in pregnancy: a randomised controlled trial. Midwifery. 2009; 25(6):649–653.
Article13. Chittumma P, Kaewkiattikun K, Wiriyasiriwach B. Comparison of the effectiveness of ginger and vitamin B6 for treatment of nausea and vomiting in early pregnancy: a randomized doubleblind controlled trial. J Med Assoc Thai. 2007; 90(1):15–20.14. Pongrojpaw D, Somprasit C, Chanthasenanont A. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. J Med Assoc Thai. 2007; 90(9):1703–1709.15. Vutyavanich T, Kraisarin T, Ruangsri R. Ginger for nausea and vomiting in pregnancy: randomized, double-masked, placebo-controlled trial. Obstet Gynecol. 2001; 97(4):577–582.
Article16. Jackson EA. Is ginger root effective for decreasing the severity of nausea and vomiting in early pregnancy? J Fam Pract. 2001; 50(8):720.17. Basirat Z, Moghadamnia A, Kashifard M, Sharifi-Razavi A. The effect of ginger biscuit on nausea and vomiting in early pregnancy. Acta Med Iran. 2009; 47(1):51–56.18. Ozgoli G, Goli M, Simbar M. Effects of ginger capsules on pregnancy, nausea, and vomiting. J Altern Complement Med. 2009; 15(3):243–246.
Article19. Fischer-Rasmussen W, Kjaer SK, Dahl C, Asping U. Ginger treatment of hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. 1991; 38(1):19–24.
Article20. Weimer K, Schulte J, Maichle A, Muth ER, Scisco JL, Horing B, Enck P, Klosterhalfen S. Effects of ginger and expectations on symptoms of nausea in a balanced placebo design. PLoS One. 2012; 7(11):e49031.
Article21. Grøntved A, Brask T, Kambskard J, Hentzer E. Ginger root against seasickness. A controlled trial on the open sea. Acta Otolaryngol. 1988; 105(1-2):45–49.22. Schmid R, Schick T, Steffen R, Tschopp A, Wilk T. Comparison of seven commonly used agents for prophylaxis of seasickness. J Travel Med. 1994; 1(4):203–206.
Article23. Regulations on statements made for dietary supplements concerning the effect of the product on the structure or function of the body. Food and Drug Administration, HHS. Final rule. Fed Regist. 2000; 65(4):1000–1050.24. Korean Intellectual Property Office. Korean traditional knowledge portal [Internet]. Daejeon: Korean Intellectual Property Office;2013. cited 2013 Nov 20. Available from: http://www.koreantk.com.25. Health Canada (CA). Natural health products ingredients database [Internet]. Ottawa: Health Canada;2013. 11. cited 2013 Sep 18. Available from: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/search-rechercheReq.do?lang=eng.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Possibility of Morning Sickness from Olfactory Hypersensitivity during Pregnancy
- The effect of ginger and metoclopramide in the prevention of nausea and vomiting during and after surgery in cesarean section under spinal anesthesia
- Common Functional Problems during Pregnancy and Association with Nutritional Status and Weight of Newborns
- Thyroid Hormone in Hyperemesis Gravidarum
- Introduction of Cybersickness