J Korean Soc Spine Surg.
2001 Dec;8(4):437-446.
Bone Forming Gene Therapy (Immune Animal Model in Ex Vivo Gene Therapy for Spinal Fusion with Type 5 Adenoviral Delivery of the LIM Mineralization Protein-1 cDNA)
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea. haksunkim@yumc.yonsei.ac.kr
- 2Department of Orthopaedic Surgery, Il-San Medical Insurance Hospital, Ko-Yang, Korea.
- 3Department of Orthopaedic Surgery, Konyang University College of Medicine, Taejeon, Korea.
- 4Emory Spine Center, Department of Orthopaedic Surgery, Emory University, Atlanta, GA, USA.
Abstract
-
STUDY DESIGN: In vivo study to determine the immune effects to adenoviral vector encoding LMP-1 cDNA in rabbit.
OBJECTIVE
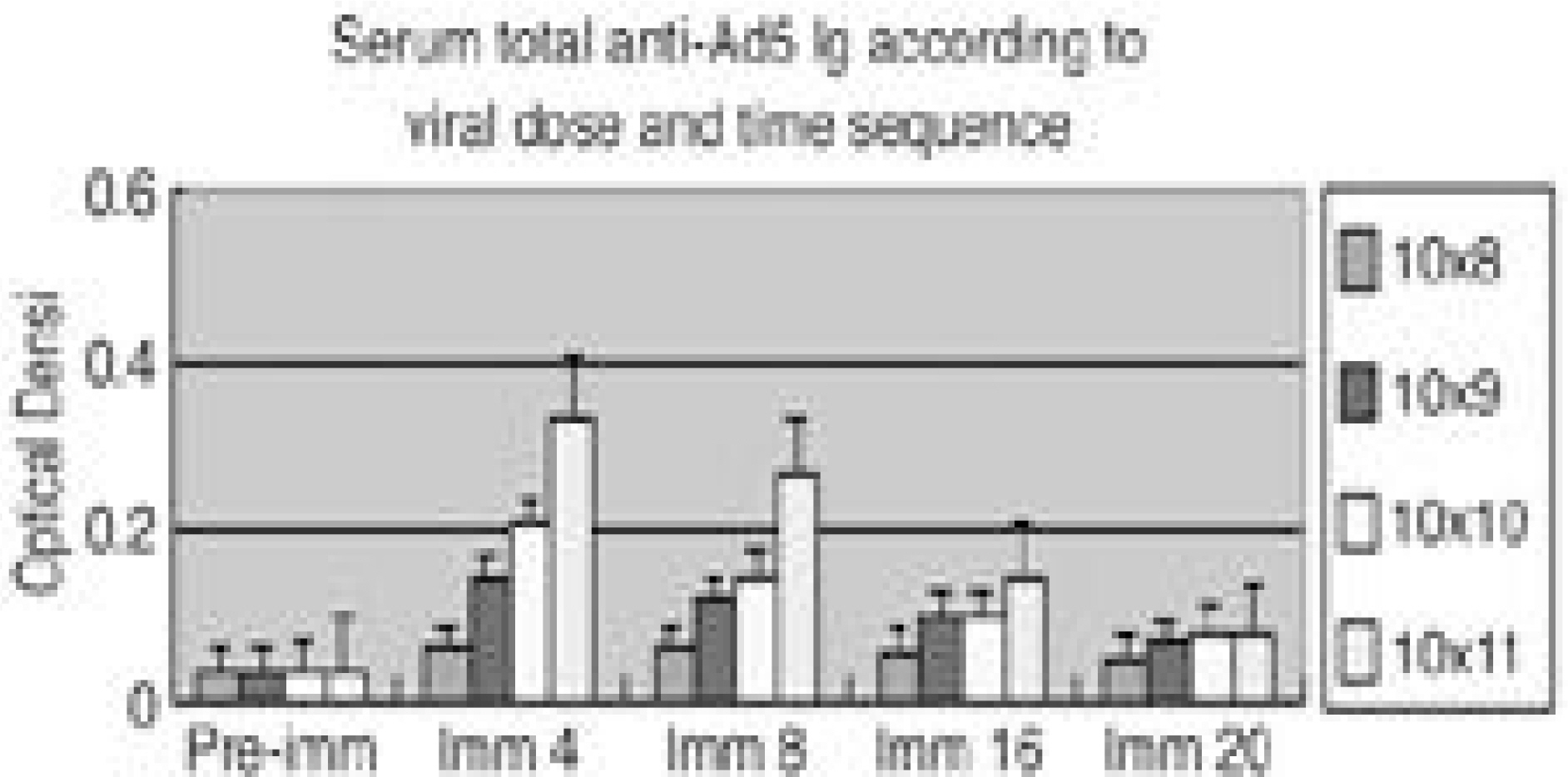
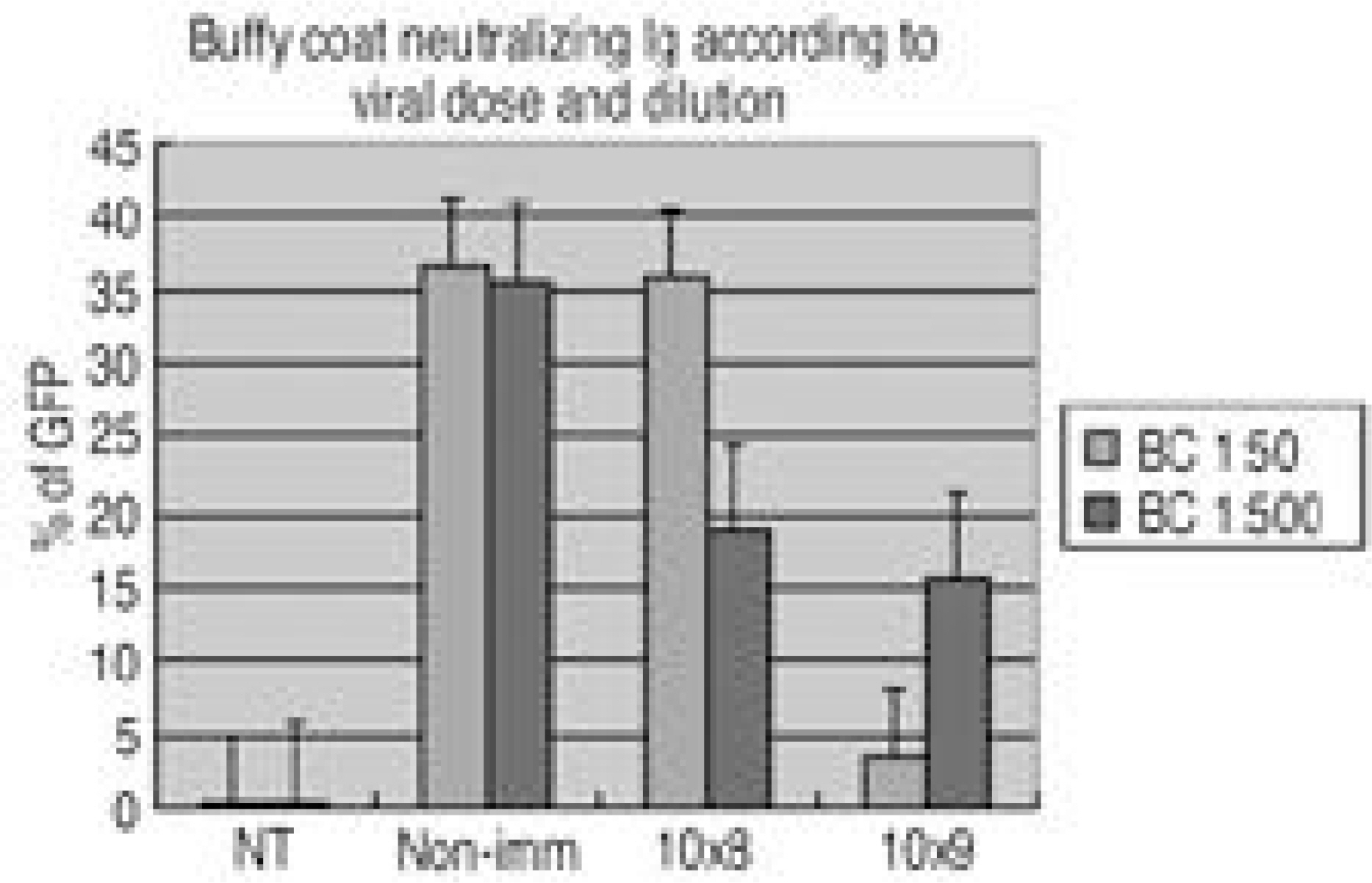
To quantify the immune effect of Ad5-LMP-1 in the rabbit during the therapeutic gene transfer. SUMMARY OF LITERATURE REVIEW: One of the major limitations in the use of adenoviral vector for gene therapy is the immune response and it made the poor transduction efficiency when re-administrated. Adenoviral antigen plus those derived from transgene expression in transduced cell contribute to cellular, humoral and non-specific immune response constitutes barriers to successful gene therapy. Therefore, the animal immune model will be mandatory to study the immune impact. MATERIALS AND METHOD: We i.v. injected Ad5-betaGal to total 24adult NZW rabbits; 1x108, 1x109, 1x1010, 1x1011v.p. to each 6 rabbits allowed them to develop immune response. Six non-immunized animals were used as control. Adenovirus antibodies were measured at 0, 4, 8, 16, 20 weeks. Group I. 6 control rabbit underwent spinal arthrodesis at 4 weeks (n=3) and 16 weeks (n=3) with 4 million cells and MOI of 4. Group II. 6 rabbit underwent spinal arthrodesis at 4 weeks after injection of 108 p.f.u virus (n=3) and 16 weeks (n=3). Group III. six 109 immunized rabbits, Group IV. six 1010 immunized rabbits, Group V. six 1011 immunized rabbits, underwent spinal arthrodesis at 4 and 16 weeks after injection. Total anti-Ad Ig and neutralizing antibody titer was measured on the 0. 4. 8, 16, 20 weeks after injection.
RESULTS
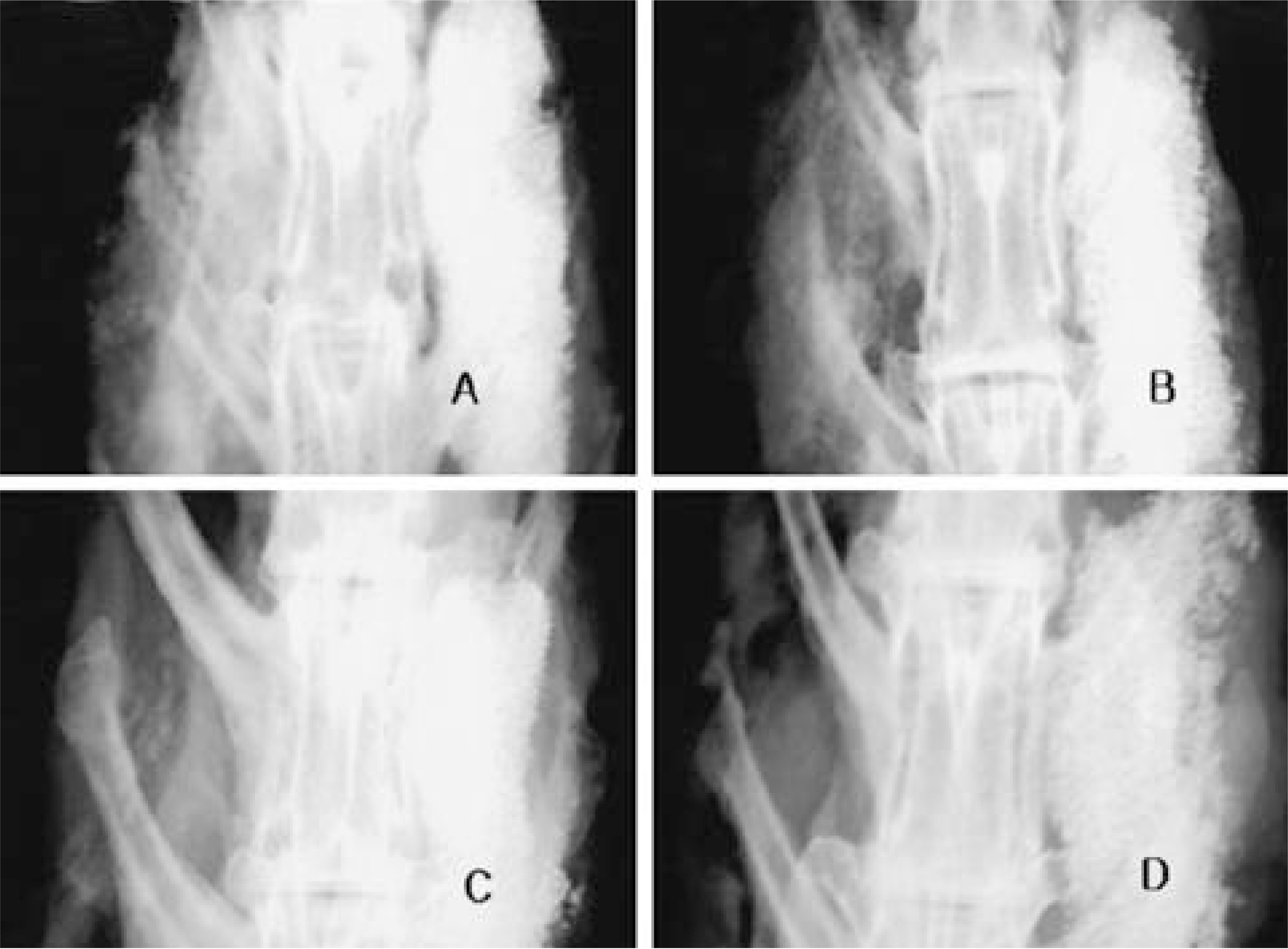
Group I. All 6 non-immunized rabbits had solid spine fusions at 4 and 16 weeks. Group II. All 3immunized rabbits had not spine fusions at 4 weeks and all three had solid spine fusion at 16 weeks. Group III. None of them (n=6) immunized rabbits had spine fusion at 4 and 16weeks, but some bone formation was observed at 16 weeks. Group IV, V. None of them immunized rabbits had bone formation. The anti-Ad5 Ig and neutralizing Ab were detected and peaked at the 4weeks and significantly dropped off 16 weeks after injection.
CONCLUSION
This experiment revealed that a small dose of adenovirus elicited an enough immune response that inhibited the bone formation. Because majority of human posses the Ab against adenovirus, it will be mandatory to overcome immune response in adenoviral vector gene therapy.
MeSH Terms
Figure
Reference
-
1). Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model: Radiographic, histologic, and biomechanical healing characteristics. Spine. 20:412–420. 1995.2). Boden SD, Schimandle JH, Hutton WC. 1995 Volvo Award in Basic Sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. Part II: Study of dose, carrier, and species. Spine. 20:2633–2644. 1995.3). Chen P, Kovesdi I, Bruder JT. Effective repeat administration with adenovirus vectors to the muscle. Gene Therapy. 7:587–595. 2000.
Article4). Chirmule N, Raper SE, Burkly L, Thomas D, Tazelaar J, Hughes JV, Wilson JM. Readministration of adenovirus vector in nonhuman primate lungs by blockade of CD40-CD40 ligand interactions. J Virol. 74:3345–3352. 2000.
Article5). Christ M, Lusky M, Stoeckel F, Dreyer D, Dieterle A, Michou AI, Pavirani A, Mehtali M. Gene therapy with recombinant adenovirus vectors: evaluation of the host immune response. Immunology Letters. 57:19–25. 1997.
Article6). Crystal R. Transfer of genes to humans: early lessons and obstacles to success. Science. 270:404–410. 1995.
Article7). Gahery-Segard H, Farace F, Godfrin D, Gaston J, Lengagne R, Tursz T, Boulanger P, Guillet JG. Immune response to recombinant capsid proteins of adenovirus in humans: antifiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J Virol. 72:2388–2397. 1998.8). Gahery-Segard H, Juillard V, Gaston J, Lengagne R, Pavirani A, Boulanger P, Guillet JG. Humoral immune response to the capsid components of recombinant adenoviruses: routes of immunization modulate virus-induced Ig subclass shifts. Eur J Immunol. 27:653–659. 1997.9). Kagami H, Atkinson JC, Michalek SM, Handelman B, Yu S, Baum BJ, O'Connell B. Repetitive adenovirus administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther. 10:305–313. 1998.
Article10). Kuriyama S, Tominaga K, Mitoro A, Tsujinoue H, Na-katani T, Yamazaki M, Nagao S, Toyokawa Y, Okamo-to S, Fukui H. Immunomodulation with FK506 around the time of intravenous re-administration of an adenoviral vector facilities gene transfer into rat liver. Int J Cancer. 85:839–844. 2000.11). Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 70:7498–7509. 1996.
Article12). Moffatt S, Hays J, HogenEsch H, Mittal SK. Circum -vention of vector specific neutralizing antibody response by alternating use of human and non-human adenoviruses: Implications in gene therapy. Virology. 272:159–167. 2000.13). Molinier-Frenkel V, Gahery-Segard H, Mehtali M, Le Boulaire C, Ribault S, Boulanger P, Tursz T, Guillet JG, Farace F. Immune response to recombinant adenovirus in humans: Capsid components from viral input are targets for vector specific cytotoxic T lymphocyte. J Virol. 74:7678–7682. 2000.14). Turner RJ, Held SDE, Hirst JE, Billinghurst G, Woot-ton RJ. An immunological assessment of group housed rabbits. Laboratory Animals. 31:362–372. 1997.15). Viggeswarapu M, Boden SD, Liu Y, Hair GA, Ugbo JL, Murakami H, Kim HS, Mayer MT, Hutton WC, Titus L. Adenoviral delivery of LIM mineralization protrin-1 induces new-bone formation in vitro and in vivo. J Bone Joint Surg Am. 83:364–376. 2001.16). Waldsworth SC, Zhou H, Smith AE, Kaplan JM. adenovirus vector-infected cells can escape adenovirus antigen specific T-lymphocyte killing in vivo. J Virol. 71:5189–5196. 1997.17). Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 91:4407–4411. 1994.
Article18). Ye X, Roinson MB, Pabin C, Batshaw ML, Wilson JM. Transient depletion of CD4 lymphocyte improves efficacy of repeated administration of recombinant adenovirus in the ornithine transcarbamylase deficient sparse fur mouse. Gene Therapy. 7:1761–1767. 2000.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spinal Fusion Based on Ex Vivo Gene Therapy Using Recombinant Human BMP Adenoviruses
- Monitoring Gene Therapy by Radionuclide Approaches
- Potentials and limitations of adenovirus-p53 gene therapy for brain tumors
- Gene Therapy and Cell Transplantation for Alzheimer's Disease and Spinal Cord Injury
- Development of New Gene Therapy for Erectile Dysfunction Using Antisense cDNA for Type V Phosphodiesterase Gene