J Korean Soc Spine Surg.
2007 Dec;14(4):235-242.
Kyphoplasty with Calcium Phosphate Cement (Calcibon(R)) in Osteoporotic Vertebral Fracture
- Affiliations
-
- 1Department of Orthopedic Surgery, Soonchunhyang University, College of Medicine, Bucheon, Korea. eungha@unitel.co.kr
Abstract
-
STUDY DESIGN: A retrospective study
OBJECTIVES
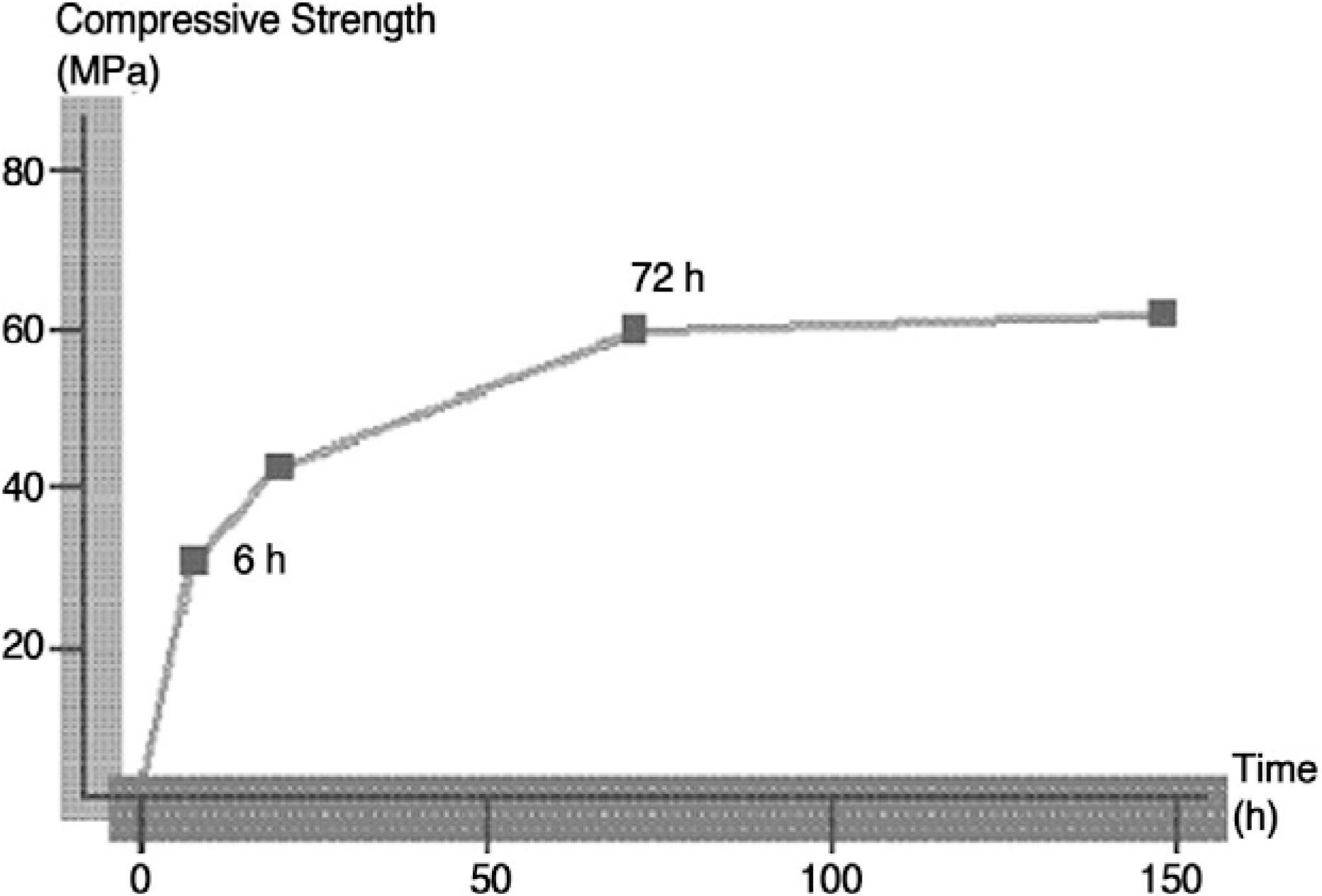
We analyzed clinical and radiological results to verify the efficacy of calcium phosphate cement in kyphoplasty for treatment of osteoporotic vertebral fracture. SUMMARY AND LITERATURE REVIEW: Calcium phosphate is a biocompatible alternative to PMMA for vertebral augmentation in painful osteoporotic vertebral fracture as it is osteoconductive, non-exothermic, and injectable.
MATERIALS AND METHODS
We analyzed 45 cases treated from April 2005 to August 2006 with a minimum of 1 year follow-up. Preoperative and post operative pain scores (visual analogue scale), ambulatory status, and patient satisfaction were measured. Anterior vertebral height, as well as the status and size of cement were assessed radiologically preoperatively, postoperatively, and at 3 months and 1 year.
RESULTS
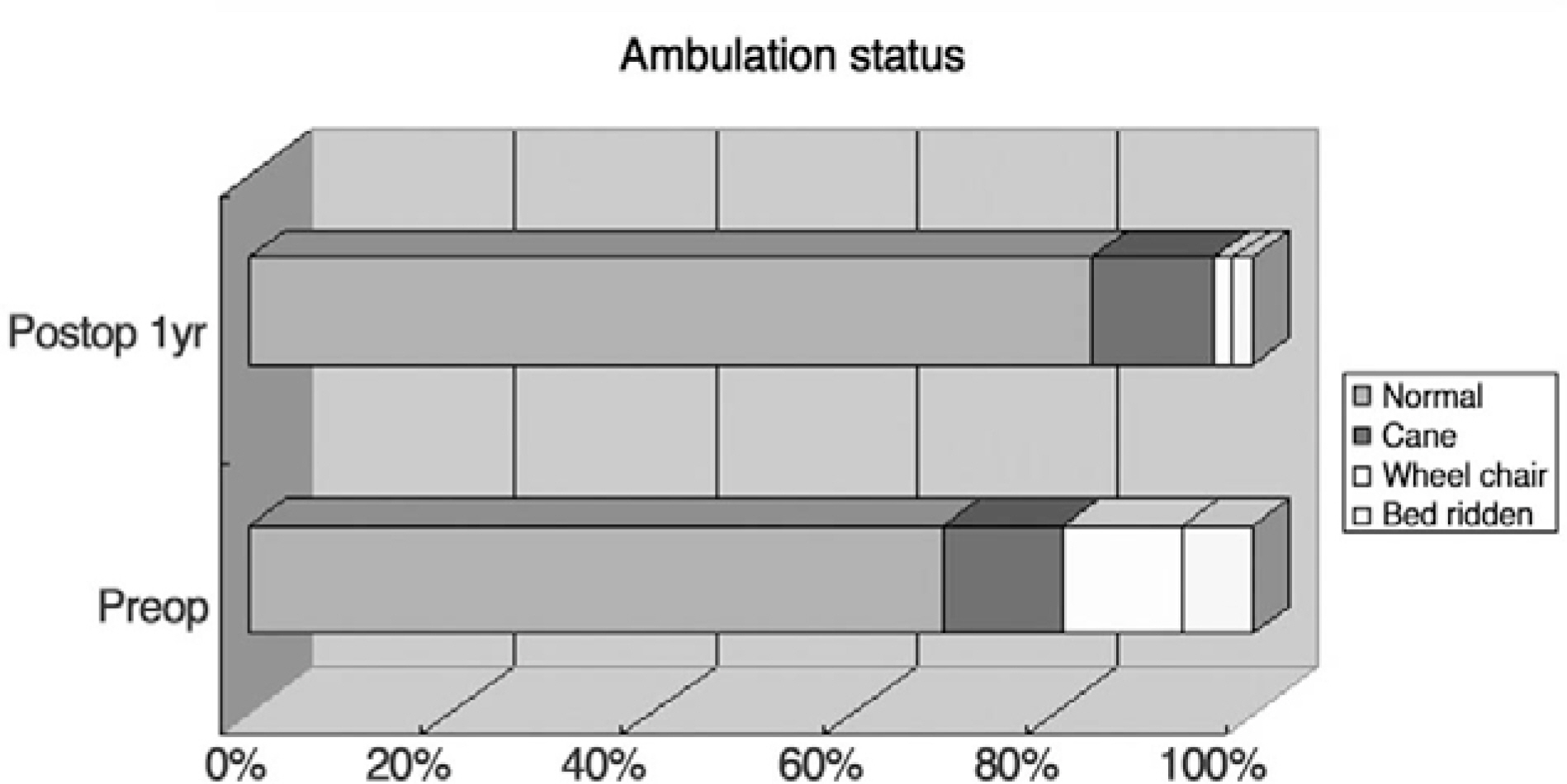
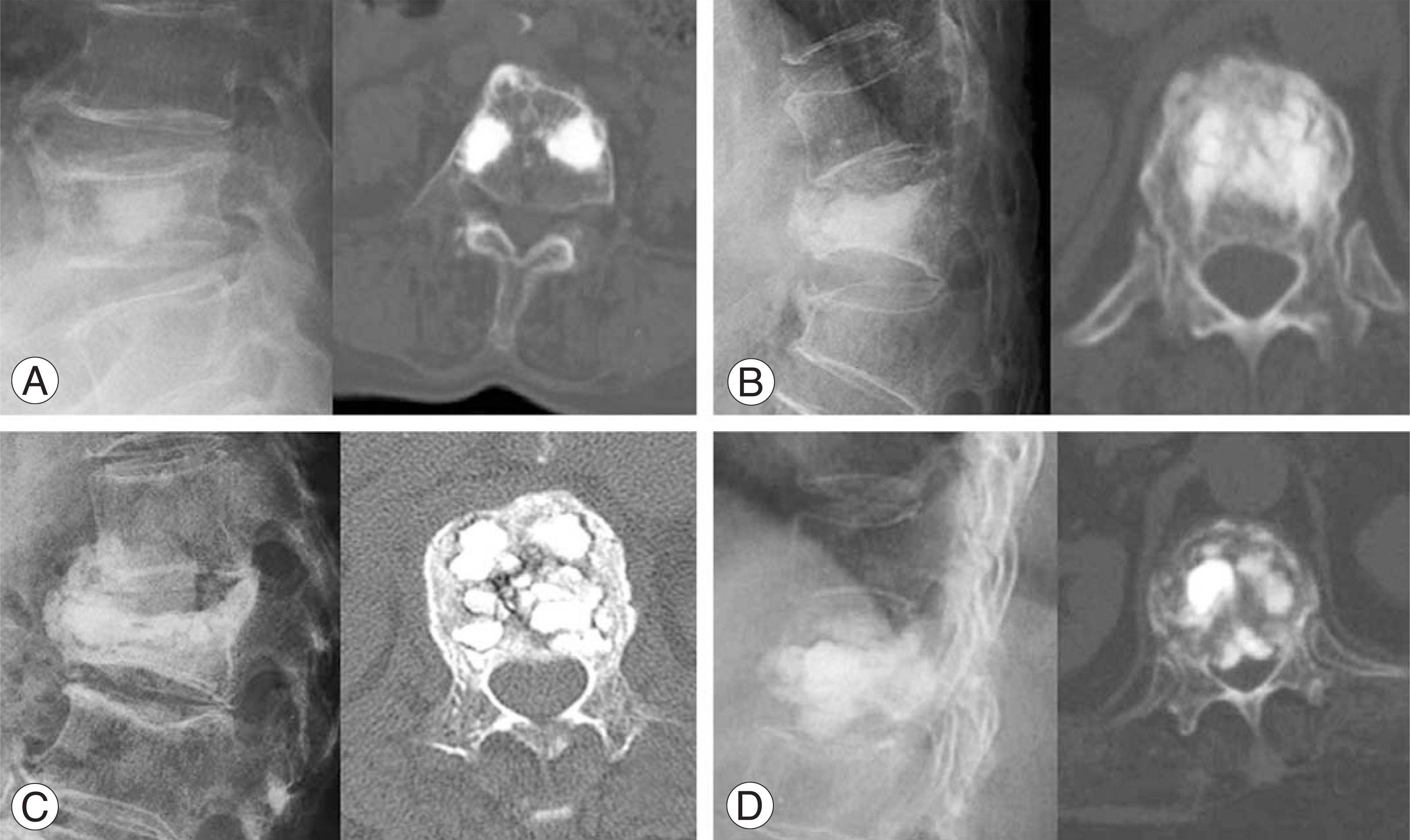
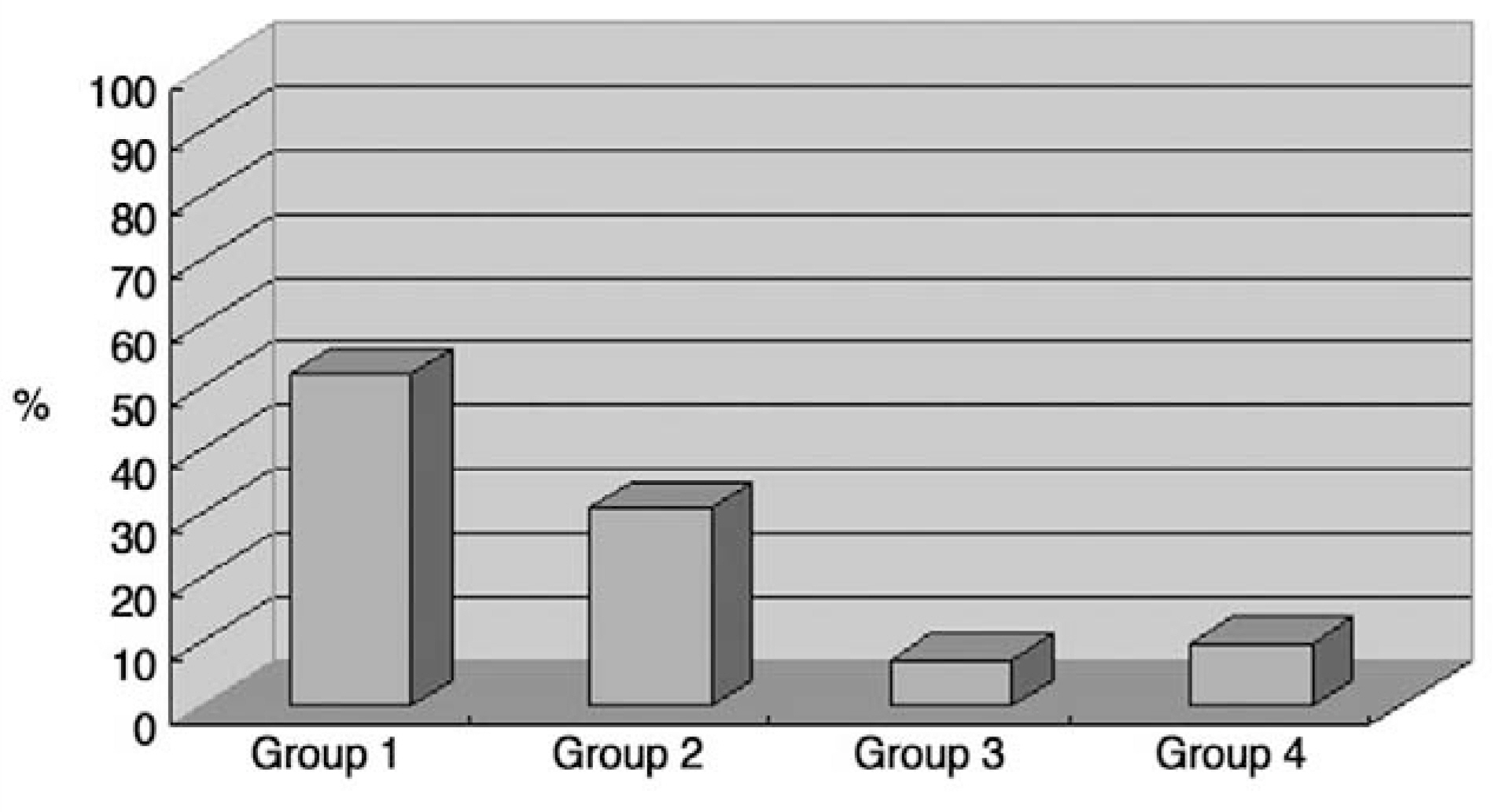
Pain scores (visual analogue scale) and ambulatory status improved significantly after kyphoplasty and remained unchanged during follow-up. Overall patient satisfaction was 93%. Radiological findings showed that mean vertebral height was significantly higher than preoperative (p<0.05). According to follow-up radiological finding, we divided cases into 4 groups: Group 1, 2; maintained vertebral height with minimal or some cement resorption; Group 3, 4; cement crack resorption and vertebral collapse. Group 1, 2 and Group 3, 4 had 38 patients (84%) and 7 patients(16%) respectively. Revision surgery was needed in 3 cases (antero-posterior surgery in 2 cases of group 4, and decompression in 1 case of extravasation into the neural canal).
CONCLUSIONS
Kyphoplasty with calcium phosphate may be a good alternative for treatment of osteoporotic vertebral fracture, but non-union of the vertebral body with a large cleft showed a high risk of premature resorption and collapse of the vertebral body. The presumed advantage over PMMA needs longer follow-up.
MeSH Terms
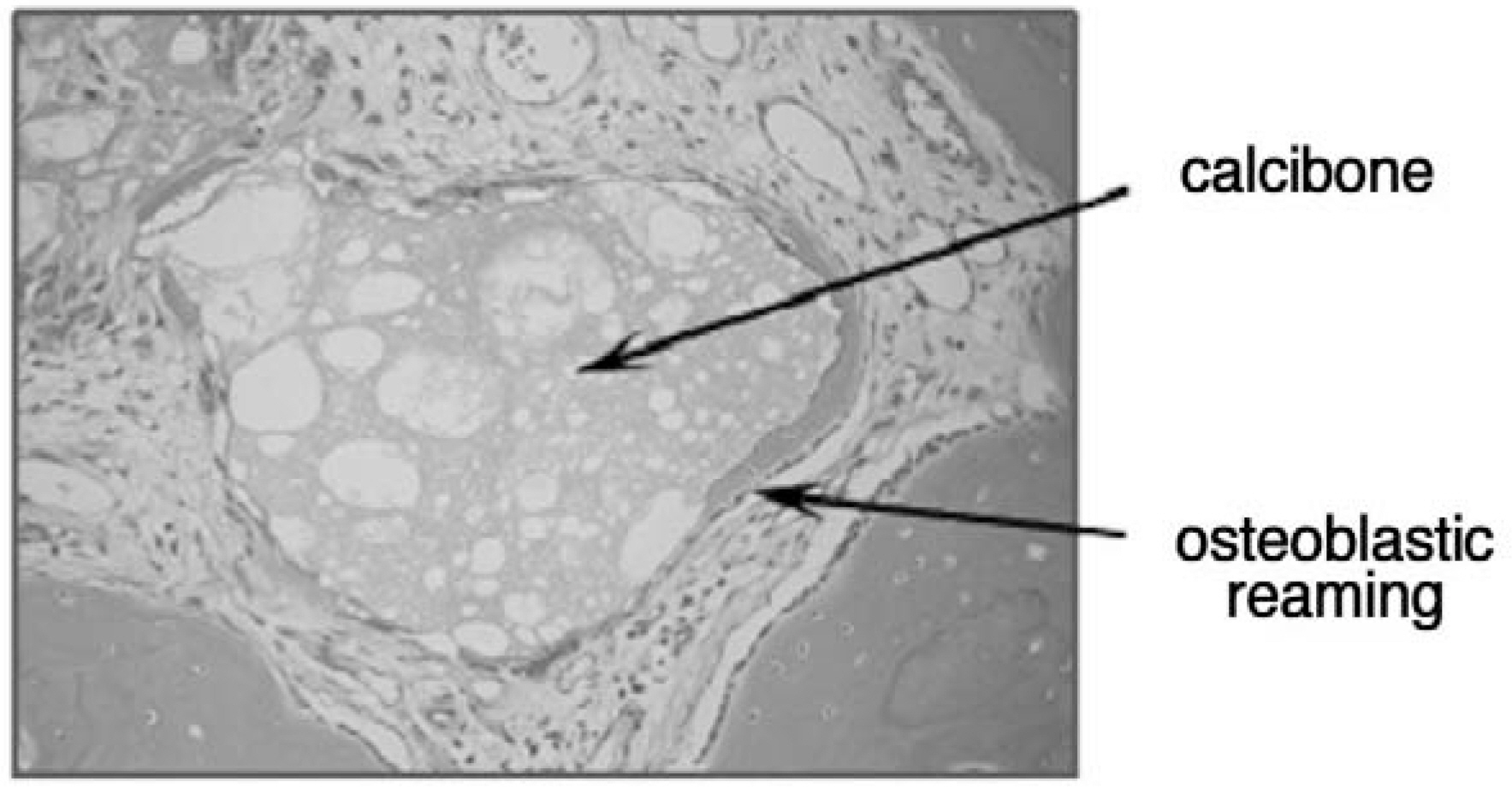
Figure
Reference
-
1). Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003; 98:21–30.
Article2). Garfin SR, Yuan HA, Reiley MA. New technologies in spine: Kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001. 1511–1515.3). Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine. 2001; 26:1631–1638.
Article4). Bai B, Jazrawi LM, Kummer FJ, Spivak JM. The use of an injectable, biodegradable calcium phosphate bone substitute for the prophylactic augmention of osteoporotic vertebrae and the management of vertebral compression fractures. Spine. 1999; 24:1521–1526.5). Ikeuchi M, Yamamoto H, Shibata T, Otani M. Mechanical augmentation of the vertebral body by calcium phosphate cement injection. J Orthop Sci. 2001; 6:39–45.
Article6). Libicher M, Hillmeier J, Liegibel U, et al. Osseous inte-gration of calcium phosphate in osteoporotic vertebral fractures after kyphoplasty: initial results from a clinical and experimental pilot study. Osteoporos Int. 2006; 17(8):1208–1215.
Article7). Grados F, Depriester C, Cayrolle G, Hardy N, Deramond H, Fardellone P. Longterm observations of vertebral osteoporotic fractures treated by percutaneous vertebroplasty. Rheumatology. 2000; 39:1410–1414.
Article8). Heini PF, Berlemann U. Bone substitutes in vertebroplasty. Eur Spine J. 2001; 10(Suppl 2):S205–S213.
Article9). Mathis JM, Barr JD, Belkoff SM, Barr MS, Jensen ME, Deramond H. Percutaneous vertebroplasty: A developing standard of care for vertebral compression fractures. AJNR Am J Neuroradiol. 2001; 22:373–381.10). Tomita S, Kin A, Yazu M, Abe M. Biomechanical evaluation of kyphoplasty and vertebroplasty with calcium phosphate cement in a simulated osteoporotic compression fracture. J Orthop Sci. 2003; 8(2):192–197.
Article11). Knaack D, Goad EB, Aiolova M, et al. Novel fully resorbable calcium phosphate bone substitute. J Bone Miner Res. 1997; 12:S202.12). Weill A, Chiras J, Simon JM, Rose M, Sola-Martinez T, Enkaoua E. Spinal metastases: Indications for and results of percutaneous injection of acrylic surgical cement. Radiology. 1996; 199:241–247.13). Deramond H, Wright NT, Belkoff SM. Temperature elevation caused by bone cement polymerization during vertebroplasty. Bone. 1999; 25:17S–21S.
Article14). Dujovny M, Aviles A, Agner C. An innovative approach for cranioplasty using hydroxyapatite cement. Surg Neurol. 1997; 48:294–297.
Article15). Knaack D, Goad EB, Aiolova M, et al. Resorbable calcium phosphate bone substitute. J Biomed Mater Res. 1998; 43:399–409.
Article16). Kurashina K, Kurita H, Hirano M, de Blieck J, Klein C, de Groot K. Calcium phosphate cement: in vitro and in vivo studies of the a-tricalcium phosphate-dicalcium phosphate dibasictetracalcium phosphate monoxide system. J Mater Sci Mater Med. 1995; 6:340–347.17). Ooms EM, Wolke JG, van der Waerden JP, Jansen JA. Trabecular bone response to injectable calcium phosphate 1 (Ca-P) cement. J Biomed Mater Res. 2002; 61(1):9–18.18). Shibata T, Yamamoto H, Iai H, et al. A biomechanical strengthening of the vertebra with osteoporosis using hydroxyapatite cement injection. J Jpn Spine Res Soc. 1995; 6:61–69.19). Ikenaga M, Hardouin P, Lemaitre J, Andrianjatovo H, Flautre B. Biomechanical characterization of a biodegradable calcium phosphate hydraulic cement: A comparison with porous biphasic calcium phosphate ceramics. J Biomed Mater Res. 1998; 40:139–144.
Article20). Pflugmacher R, Schroeder RJ, Klostermann CK. Incidence of adjacent vertebral fractures in patients treated with balloon kyphoplasty: two years’ prospective followup. Acta Radiol. 2006; 47(8):830–840.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Evaluation of Calcium Phosphate Cement Compared with Polymethylmethacrylate for Kyphoplasty
- Asymptomatic Pulmonary Embolus Following Percutaneous Kyphoplasty: A Case Report
- Minimally Invasive Treatment of Painful Osteoporotic Vertebral Fractures
- Successful Bone Union Following Calcium Phosphate Cement-Assisted Percutaneous Transpedicular Balloon Kyphoplasty of a Large Interbody Cleft on Long-term Hemodialysis Patient
- Cement Leakage into Disc after Kyphoplasty: Does It Increases the Risk of New Adjacent Vertebral Fractures?