World J Mens Health.
2014 Dec;32(3):159-166. 10.5534/wjmh.2014.32.3.159.
Primary Androgen Deprivation Therapy for Prostate Cancer in Koreans: A Retrospective Multicenter Study
- Affiliations
-
- 1Department of Urology, Inje University College of Medicine, Busan, Korea. urokang@lycos.co.kr
- 2Department of Urology, Dong-A University College of Medicine, Busan, Korea.
- 3Department of Urology, University of Ulsan College of Medicine, Ulsan, Korea.
- 4Department of Urology, Kosin University College of Medicine, Busan, Korea.
- 5Department of Urology, Ewha Womans University School of Medicine, Seoul, Korea.
- 6Rutgers Cancer Institute of New Jersey, Rutgers, The State University of New Jersey, New Brunswick, NJ, USA.
- KMID: 2320780
- DOI: http://doi.org/10.5534/wjmh.2014.32.3.159
Abstract
- PURPOSE
To evaluate the characteristics of patients who received primary androgen deprivation therapy (PADT) for prostate cancer and the clinical efficacy of this treatment.
MATERIALS AND METHODS
Two hundred forty patients treated by PADT were reviewed. These patients could not receive definitive therapy owing to old age, patient need, and medical comorbidity. The patients were divided into three groups according to the extent of prostate cancer: localized, locally advanced, and metastatic. Then, prostate-specific antigen (PSA) progression in these groups was analyzed.
RESULTS
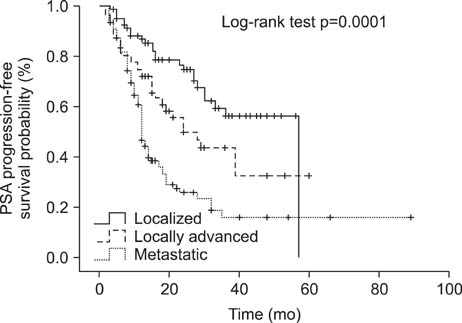
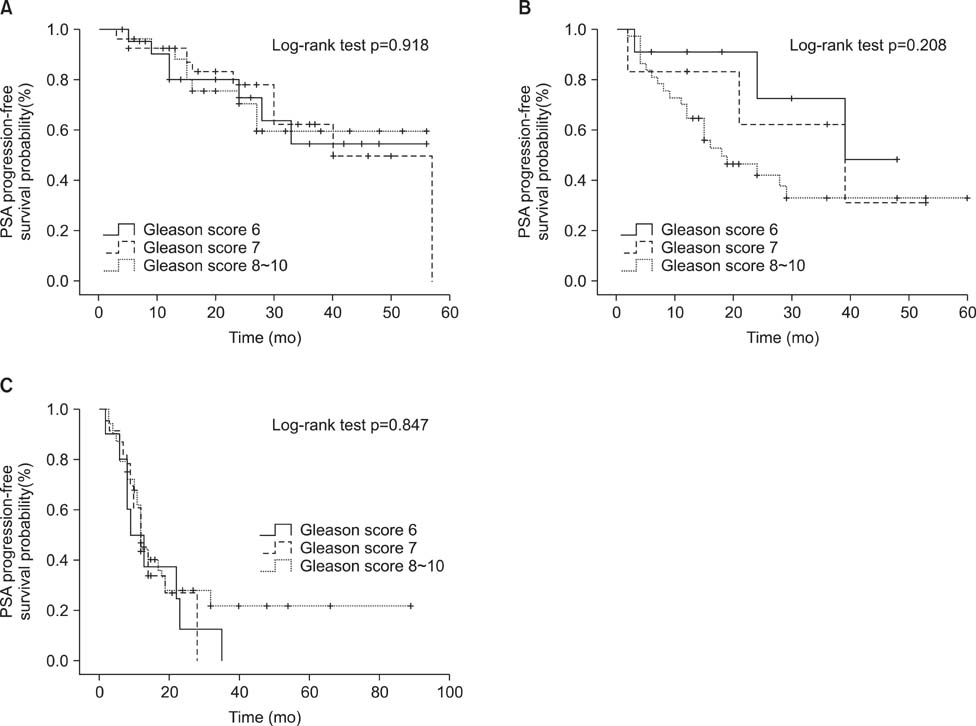
The median age of the patients was 73.0 years, and the median pretreatment PSA level was 47.0 ng/mL. Of the patients, 91.7% were treated with combined androgen blockade, and 8.3% were treated with monotherapy. Clinical factors for PSA progression were a PSA nadir and a high clinical stage. Estimated PSA recurrence-free median survival time in each group was 57, 24, and 12 months, respectively. A PSA nadir of >0.2 ng/mL and metastatic stage were independent factors for expecting a poor response to PADT (hazard ratio 4.26, p<0.001; and 2.60, p<0.001).
CONCLUSIONS
Patients with localized or locally advanced prostate cancer who did not receive definitive therapy had lower PSA progression rates than those at metastatic stage during PADT. Further, a PSA nadir of < or =0.2 ng/mL showed better progression-free survival. Therefore, PADT can be another therapeutic option in well-selected patients with localized or locally advanced prostate cancer and PSA change should be checked carefully.
Keyword
MeSH Terms
Figure
Reference
-
1. Huggins C, Hodges CV. Studies in prostate cancer: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of prostate. Cancer Res. 1941; 1:293–297.2. Chedin M, Filhol O, Duminy C, Bolla M, Benistant C, Roche S, et al. Characterization of two different cytoplasmic protein tyrosine kinases from human breast cancer. Carcinogenesis. 1997; 18:1463–1472.
Article3. Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002; 360:103–106.
Article4. Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999; 341:1781–1788.
Article5. D'Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004; 292:821–827.6. Schröder FH, Kurth KH, Fosså SD, Hoekstra W, Karthaus PP, Debois M, et al. Members of the European Organisation for the Research and Treatment of Cancer Genito-urinary Group. Early versus delayed endocrine treatment of pN1-3 M0 prostate cancer without local treatment of the primary tumor: results of European Organisation for the Research and Treatment of Cancer 30846: a phase III study. J Urol. 2004; 172:923–927.7. Pagliarulo V, Bracarda S, Eisenberger MA, Mottet N, Schröder FH, Sternberg CN, et al. Contemporary role of androgen deprivation therapy for prostate cancer. Eur Urol. 2012; 61:11–25.
Article8. Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003; 95:981–989.
Article9. Janoff DM, Peterson C, Mongoue-Tchokote S, Peters L, Beer TM, Wersinger EM, et al. Clinical outcomes of androgen deprivation as the sole therapy for localized and locally advanced prostate cancer. BJU Int. 2005; 96:503–507.
Article10. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Chapter 3. Tumours of the Prostate [Internet]. Lyon (France): IARC Press;2004. cited 2014 Jul 15. Available from: http://www.iarc.fr/en/publications/pdfs-online/pat-gen/bb7/bb7-chap3.pdf.11. Kawakami J, Cowan JE, Elkin EP, Latini DM, DuChane J, Carroll PR. CaPSURE Investigators. Androgen-deprivation therapy as primary treatment for localized prostate cancer: data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE). Cancer. 2006; 106:1708–1714.12. DiBlasio CJ, Malcolm JB, Hammett J, Wan JY, Aleman MA, Patterson AL, et al. Survival outcomes in men receiving androgen-deprivation therapy as primary or salvage treatment for localized or advanced prostate cancer: 20-year single-centre experience. BJU Int. 2009; 104:1208–1214.
Article13. Akaza H, Homma Y, Usami M, Hirao Y, Tsushima T, Okada K, et al. Prostate Cancer Study Group. Efficacy of primary hormone therapy for localized or locally advanced prostate cancer: results of a 10-year follow-up. BJU Int. 2006; 98:573–579.
Article14. Lu-Yao GL, Albertsen PC, Li H, Moore DF, Shih W, Lin Y, et al. Does primary androgen-deprivation therapy delay the receipt of secondary cancer therapy for localized prostate cancer? Eur Urol. 2012; 62:966–972.
Article15. Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997; 57:314–319.16. Koivisto PA, Helin HJ. Androgen receptor gene amplification increases tissue PSA protein expression in hormone-refractory prostate carcinoma. J Pathol. 1999; 189:219–223.
Article17. Shi Y, Brands FH, Chatterjee S, Feng AC, Groshen S, Schewe J, et al. Her-2/neu expression in prostate cancer: high level of expression associated with exposure to hormone therapy and androgen independent disease. J Urol. 2001; 166:1514–1519.
Article18. Ricciardelli C, Jackson MW, Choong CS, Stahl J, Marshall VR, Horsfall DJ, et al. Elevated levels of HER-2/neu and androgen receptor in clinically localized prostate cancer identifies metastatic potential. Prostate. 2008; 68:830–838.19. Jennbacken K, Tesan T, Wang W, Gustavsson H, Damber JE, Welén K. N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr Relat Cancer. 2010; 17:469–479.
Article20. Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008; 300:173–181.
Article21. Kim TS, Kang SH, Rhew HY. Efficacy of androgen deprivation therapy in patients with clinically localized prostate cancer. Korean J Urol. 2009; 50:1073–1077.
Article22. Tanaka N, Hirayama A, Yoneda T, Yoshida K, Shimada K, Konishi N, et al. Trends of risk classification and primary therapy for Japanese patients with prostate cancer in Nara Uro-Oncological Research Group (NUORG): a comparison between 2004-2006 and 2007-2009. BMC Cancer. 2013; 13:588.
Article23. Akaza H. Asian trends in primary androgen depletion therapy on prostate cancer. Cancer Biol Med. 2013; 10:187–191.24. Bill-Axelson A, Holmberg L, Ruutu M, Häggman M, Andersson SO, Bratell S, et al. Scandinavian Prostate Cancer Group Study No. 4. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005; 352:1977–1984.
Article25. Situmorang GR, Umbas R, Mochtar CA, Santoso RB. Prostate cancer in younger and older patients: do we treat them differently? Asian Pac J Cancer Prev. 2012; 13:4577–4580.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Current Concepts in Androgen Deprivation Therapy
- Intermittent Androgen Deprivation with Goserelin and Flutamide for Prostate Cancer: a Pilot Study
- Long-Lasting Antiandrogen Withdrawal Syndrome in Castration-Resistant Prostate Cancer: Three Cases With Complete Response
- Differentiation Related Gene (Drg-1) as a Molecular Marker during the Treatment of in vitro Intermittent Androgen Deprivation in prostate Cancer