Tuberc Respir Dis.
2009 Sep;67(3):221-225.
Pain Management Based on NCCN Guideline in Patients with Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Korea Cancer Center Hospital, Korea Institute of Radiological & Medical Sciences, Seoul, Korea. jclee@kcch.re.kr
Abstract
- BACKGROUND
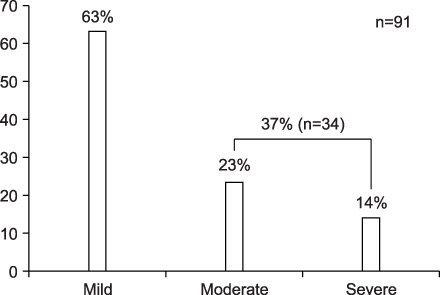
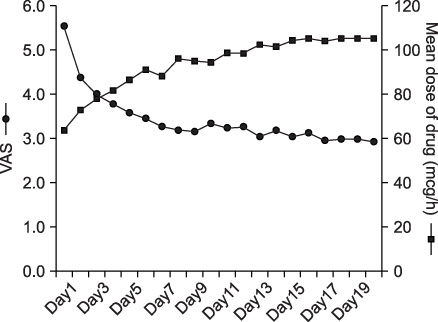
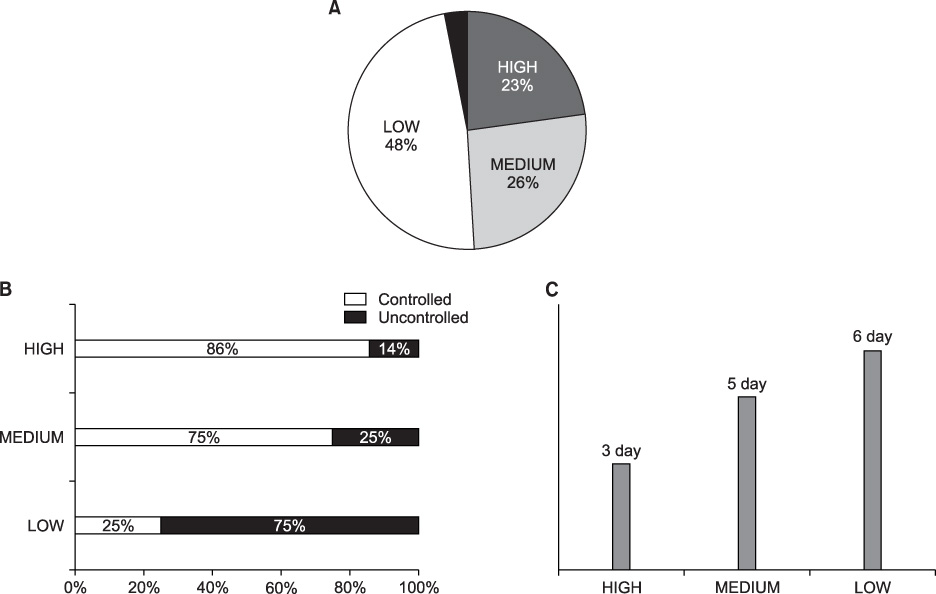
Pain is one of the most troublesome problems caused by malignancy. We evaluated the change in pain status according to observance of NCCN guidelines in lung cancer patients. METHODS: Lung cancer patients complaining of pain at admission were examined. The pain was assessed with visual analogue scale (VAS) for 20 days and moderate-to-severe pain was defined as more than VAS level 3. The guideline observance was classified as high (more than 80%), medium (50~79%) and low (less than 50%). RESULTS: Among the total 91 lung cancer patients with pain, 34 patients (37%) had moderate-to-severe pain. Their average VAS score at admission was 5.6. It decreased to 2.9 after a 20-day period of pain management. The time to reach a VAS less than 3 was 3 days in a high guideline observance group, while it took 6 days in a low observance group. In addition, the pain in the high observance group was controlled to less than 3 VAS level in 86% of patients, whereas only 25% of patients in the low observance group succeeded. CONCLUSION: Pain was more effectively controlled when the dose of drugs was modified according to NCCN guidelines in lung cancer patients indicating the importance of guideline observance in pain management.
Keyword
MeSH Terms
Figure
Reference
-
1. Korean Society for Hospice and Palliative Care, Korean Cancer Study Group. Cancer pain relief guideline. 2001. Seoul: Korean Society for Hospice and Palliative Care, Korean Cancer Study Group.2. Heo DS. Symptom control. J Korean Med Assoc. 1998. 41:1125–1130.3. Yun YH, Kim CH. Resident's knowledge and attitude towards cancer pain management. J Korean Acad Fam Med. 1997. 18:591–600.4. NCCN clinical practice guidelines in oncology: adult cancer pain [Internet]. National Comprehensive Cancer Network. 2008. Washington: National Comprehensive Cancer Network;Available from: http://www.nccn.org/.5. Ahles TA, Blanchard EB, Ruckdeschel JC. The multidimensional nature of cancer-related pain. Pain. 1983. 17:277–288.6. Daut RL, Cleeland CS. The prevalence and severity of pain in cancer. Cancer. 1982. 50:1913–1918.7. American Pain Society Quality of Care Committee. Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995. 274:1874–1880.8. Ventafridda V, Tamburini M, Caraceni A, De Conno F, Naldi F. A validation study of the WHO method for cancer pain relief. Cancer. 1987. 59:850–856.9. Grond S, Zech D, Schug SA, Lynch J, Lehmann KA. Validation of World Health Organization guidelines for cancer pain relief during the last days and hours of life. J Pain Symptom Manage. 1991. 6:411–422.10. Zech DF, Grond S, Lynch J, Hertel D, Lehmann KA. Validation of World Health Organization Guidelines for cancer pain relief: a 10-year prospective study. Pain. 1995. 63:65–76.11. Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999. 353:1695–1700.12. Kim KH, Jang WI, Joh YH, Choi IS, Park SR, Lee SY, et al. Evaluation of the adequacy of pain management in the admitted cancer patients. Korean J Hosp Palloat Care. 2001. 4:137–144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pilot Study on Development of Telecommunication Guideline for Symptom Management of Lung Cancer Patients
- Guidelines for the Management of Postoperative Pain after Total Knee Arthroplasty
- Development of the Nursing Practice Guideline for Pain Management according to the Guideline Adaptation Process
- Development and Usability Test of a Website for Cancer Symptom Management
- 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma