Tuberc Respir Dis.
2008 Sep;65(3):177-182.
Standard Chemotherapy with Excluding Isoniazid in a Murine Model of Tuberculosis
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Korea. shimts@amc.seoul.kr
- 2Department of Microbiology and the Brain Korea 21 Project for Medical Sciences, Yonsei University College of Medicine, Seoul, Korea.
Abstract
-
BACKGROUND: Isoniazid (INH, H) is a key drug of the standard first-line regimen for the treatment of tuberculosis (TB), yet some reports have suggested that treatment efficacy was maintained even though INH was omitted from the treatment regimen.
METHODS
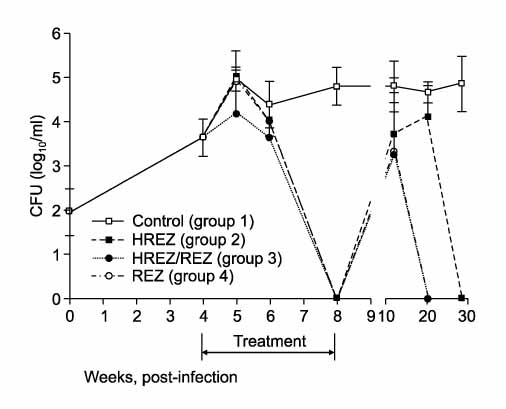
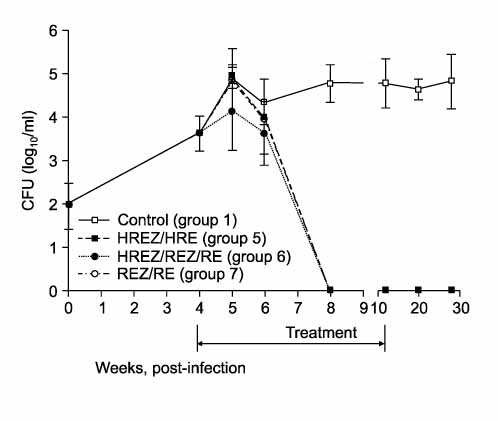
One hundred forty C57BL/6 mice were infected with the H37Rv strain of M. tuberculosis with using a Glas-Col aerosol generation device, and this resulted in depositing about 100 bacilli in the lung. Four weeks after infection, anti-TB treatment was initiated with varying regimens for 4-8 weeks; Group 1: no treatment (control), Group 2 (4HREZ): 4 weeks of INH, rifampicin (R), pyrazinamide (Z) and ethambutol (E), Group 3: 1HREZ/3REZ, Group 4: 4REZ, Group 5: 4HREZ/4HRE, Group 6: 1HREZ/3REZ/4RE, and Group 7: 4REZ/4RE. The lungs and spleens were harvested at several time points until 28 weeks after infection, and the colony-forming unit (CFU) counts were determined.
RESULTS
The CFU counts increased steadily after infection in the control group. In the 4-week treatment groups (Group 2-4), even though the culture was negative at treatment completion, the bacilli grew again at the 12-week and 20-week time points after completion of treatment. In the 8-week treatment groups (Groups 5-7), the bacilli did not grow in the lung at 4 weeks after treatment initiation and thereafter. In the spleens of Group 7 in which INH was omitted from the treatment regimen, the culture was negative at 4-weeks after treatment initiation and thereafter. However, in Groups 5 and 6 in which INH was taken continuously or intermittently, the bacilli grew in the spleen at some time points after completion of treatment.
CONCLUSION
TThe exclusion of INH from the standard first-line regimen did not affect the treatment outcome in a murine model of TB in the early stage of disease. Further studies using a murine model of chronic TB are necessary to clarify the role of INH in the standard first-line regimen for treating TB.
Keyword
MeSH Terms
Figure
Reference
-
1. American Thoracic Society; Centers for Disease Control and Prevention; Infectious Diseases Society of America. Treatment of tuberculosis. MMWR Recomm Rep. 2003. 52:1–77.2. Bai GH. Anti-tuberculosis drug resistance in Korea. Commun Dis Month Rep. 2005. 16:101–107.3. Mitchison DA, Nunn AJ. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis. 1986. 133:423–430.4. Lecoeur HF, Truffot-Pernot C, Grosset JH. Experimental short-course preventive therapy of tuberculosis with rifampin and pyrazinamide. Am Rev Respir Dis. 1989. 140:1189–1193.5. Grosset J, Truffot-Pernot C, Lacroix C, Ji B. Antagonism between isoniazid and the combination pyrazinamiderifampin against tuberculosis infection in mice. Antimicrob Agents Chemother. 1992. 36:548–551.6. Jindani A, Dore CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003. 167:1348–1354.7. Nuermberger EL, Yoshimatsu T, Tyagi S, O'Brien RJ, Vernon AN, Chaisson RE, et al. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am J Respir Crit Care Med. 2004. 169:421–426.8. Rosenthal IM, Williams K, Tyagi S, Vernon AA, Peloquin CA, Bishai WR, et al. Weekly moxifloxacin and rifapentine is more active than the denver regimen in murine tuberculosis. Am J Respir Crit Care Med. 2005. 172:1457–1462.9. Roberts AD, Cooper AM, Beslisle JT, Turner J, Gonzalez-Juarrero M, Orme IM. Kaufmann SHE, Kabelitz D, editors. Murine model of tuberculosis. Methods in microbiology. 2002. 32. New York: Elsevier Sciences;433.10. Nuermberger E, Rosenthal I, Tyagi S, Williams KN, Almeida D, Peloquin CA, et al. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob Agents Chemother. 2006. 50:2621–2625.11. Centers for Disease Control and Prevention. Fatal and severe hepatitis associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection--New York and Georgia, 2000. MMWR Morb Mortal Wkly Rep. 2001. 50:289–291.12. Centers for Disease Control and Prevention. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection-United States, 2003. MMWR Morb Mortal Wkly Rep. 2003. 52:735–739.13. Centers for Disease Control and Prevention. Update: Fatal and severe liver injuries associated with rifampin and pyrazinamide treatment for latent tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2002. 51:998–999.