Restor Dent Endod.
2016 Feb;41(1):12-21. 10.5395/rde.2016.41.1.12.
Effect of acidic solutions on the microhardness of dentin and set OrthoMTA and their cytotoxicity on murine macrophage
- Affiliations
-
- 1Department of Conservative Dentistry, Dental Research Institute, Seoul National University Dental Hospital, Seoul National University School of Dentistry, Seoul, Korea. kum6139@snu.ac.kr
- 2Schulich School of Medicine and Dentistry, University of Western Ontario, London, Canada.
- 3Department of Conservative Dentistry, Wonju Severance Christian Hospital, Yonsei University, Wonju, Korea.
- 4Bendilde St. Margaret's School, St. Louis Park, MN, USA.
- 5Department of Conservative Dentistry, School of Dentistry, Kyung Hee University, Seoul, Korea.
- KMID: 2316959
- DOI: http://doi.org/10.5395/rde.2016.41.1.12
Abstract
OBJECTIVES
To evaluate the effects of three acids on the microhardness of set mineral trioxide aggregate (MTA) and root dentin, and cytotoxicity on murine macrophage.
MATERIALS AND METHODS
OrthoMTA (BioMTA) was mixed and packed into the human root dentin blocks of 1.5 mm diameter and 5 mm height. Four groups, each of ten roots, were exposed to 10% citric acid (CA), 5% glycolic acid (GA), 17% ethylenediaminetetraacetic acid (EDTA), and saline for five minutes after setting of the OrthoMTA. Vickers surface microhardness of set MTA and dentin was measured before and after exposure to solutions, and compared between groups using one-way ANOVA with Tukey test. The microhardness value of each group was analyzed using student t test. Acid-treated OrthoMTA and dentin was examined by scanning electron microscope (SEM). Cell viability of tested solutions was assessed using WST-8 assay and murine macrophage.
RESULTS
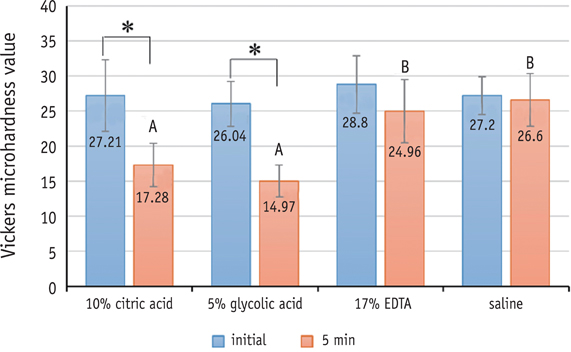
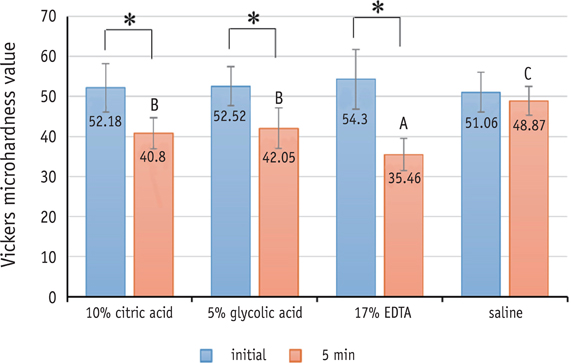
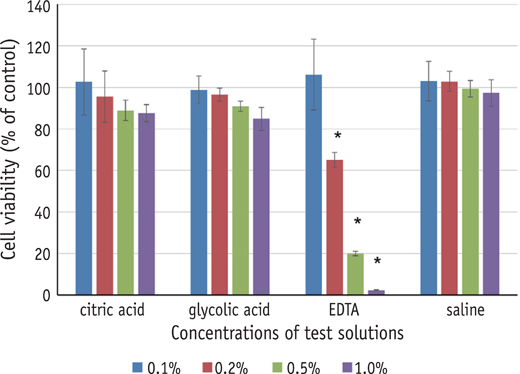
Three test solutions reduced microhardness of dentin. 17% EDTA demonstrated severe dentinal erosion, significantly reduced the dentinal microhardness compared to 10% CA (p = 0.034) or 5% GA (p = 0.006). 10% CA or 5% GA significantly reduced the surface microhardness of set MTA compared to 17% EDTA and saline (p < 0.001). Acid-treated OrthoMTA demonstrated microporous structure with destruction of globular crystal. EDTA exhibited significantly more cellular toxicity than the other acidic solutions at diluted concentrations (0.2, 0.5, 1.0%).
CONCLUSIONS
Tested acidic solutions reduced microhardness of root dentin. Five minute's application of 10% CA and 5% GA significantly reduced the microhardness of set OrthoMTA with lower cellular cytotoxicity compared to 17% EDTA.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Carbohydrate-electrolyte drinks exhibit risks for human enamel surface loss
Mary Anne Sampaio de Melo, Vanara Florêncio Passos, Juliana Paiva Marques Lima, Sérgio Lima Santiago, Lidiany Karla Azevedo Rodrigues
Restor Dent Endod. 2016;41(4):246-254. doi: 10.5395/rde.2016.41.4.246.
Reference
-
1. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993; 19:591–595.
Article2. Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007; 40:462–470.
Article3. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review-part II: leakage and biocompatibility investigations. J Endod. 2010; 36:190–202.
Article4. Li Z, Cao L, Fan M, Xu Q. Direct pulp capping with calcium hydroxide or mineral trioxide aggregate: a meta-analysis. J Endod. 2015; 41:1412–1417.
Article5. Bogen G, Kuttler S. Mineral trioxide aggregate obturation: a review and case series. J Endod. 2009; 35:777–790.
Article6. Lee BN, Moon JW, Chang HS, Hwang IN, Oh WM, Hwang YC. A review of the regenerative endodontic treatment procedure. Restor Dent Endod. 2015; 40:179–187.
Article7. Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB. Challenges in regenerative endodontics: a case series. J Endod. 2010; 36:536–541.
Article8. Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007; 33:377–390.
Article9. Nosrat A, Homayounfar N, Oloomi K. Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: a literature review and report of a case. J Endod. 2012; 38:1428–1434.
Article10. Felman D, Parashos P. Coronal tooth discoloration and white mineral trioxide aggregate. J Endod. 2013; 39:484–487.
Article11. Boutsioukis C, Noula G, Lambrianidis T. Ex vivo study of the efficiency of two techniques for the removal of mineral trioxide aggregate used as a root canal filling material. J Endod. 2008; 34:1239–1242.
Article12. Saghiri MA, Garcia-Godoy F, Gutmann JL, Sheibani N, Asatourian A, Lotfi M, Elyasi M. Removal of white mineral trioxide aggregate cement: a promising approach. Biomed Res Int. 2013; 2013:469164.
Article13. Nandini S, Natanasabapathy V, Shivanna S. Effect of various chemicals as solvents on the dissolution of set white mineral trioxide aggregate: an in vitro study. J Endod. 2010; 36:135–138.
Article14. Kayahan MB, Nekoofar MH, Kazandağ M, Canpolat C, Malkondu O, Kaptan F, Dummer PM. Effect of acidetching procedure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2009; 42:1004–1014.
Article15. Butt N, Talwar S. In vitro evaluation of various solvents for retrieval of mineral trioxide aggregate and their effect on microhardness of dentin. J Conserv Dent. 2013; 16:199–202.
Article16. Lee YL, Lin FH, Wang WH, Ritchie HH, Lan WH, Lin CP. Effects of EDTA on the hydration mechanism of mineral trioxide aggregate. J Dent Res. 2007; 86:534–538.
Article17. Batavia P, Parekh V, Batavia P, Kothari P, Chappla H, Dabhi M. Comparative evaluation of effect of three different mineral trioxide aggregate solvents on calcium content of root dentin: an in vitro study. J Interdiscip Dent. 2014; 4:8–12.
Article18. Mariño FT, Torres J, Hamdan M, Rodríguez CR, Cabarcos EL. Advantages of using glycolic acid as a retardant in a brushite forming cement. J Biomed Mater Res B Appl Biomater. 2007; 83:571–579.
Article19. Ramachandran VS, Lowery MS. Conduction calorimetric investigation of the effect of retarders on the hydration of portland cement. Thermochim Acta. 1992; 195:373–387.
Article20. The Chemours company. Glycolic acid. updated 2015 Oct 02. Available from: https://www.chemours.com/Glycolic_Acid/en_US/uses_apps/cleaning/cleaning_pgs/concrete_cleaners.html.21. Kum KY, Zhu Q, Safavi K, Gu Y, Bae KS, Chang SW. Analysis of six heavy metals in Ortho mineral trioxide aggregate and ProRoot mineral trioxide aggregate by inductively coupled plasma-optical emission spectrometry. Aust Endod J. 2013; 39:126–130.
Article22. Yoo JS, Chang SW, Oh SR, Perinpanayagam H, Lim SM, Yoo YJ, Oh YR, Woo SB, Han SH, Zhu Q, Kum KY. Bacterial entombment by intratubular mineralization following orthograde mineral trioxide aggregate obturation: a scanning electron microscopy study. Int J Oral Sci. 2014; 6:227–232.
Article23. Kim SY, Kim KJ, Yi YA, Seo DG. Quantitative microleakage analysis of root canal filling materials in single-rooted canals. Scanning. 2015; 37:237–245.
Article24. Yamada RS, Armas A, Goldman M, Lin PS. A scanning electron microscopic comparison of a high volume final flush with several irrigating solutions: part 3. J Endod. 1983; 9:137–142.
Article25. Arends J, ten Bosch JJ. Demineralization and remineralization evaluation techniques. J Dent Res. 1992; 71:924–928.
Article26. Pashley D, Okabe A, Parham P. The relationship between dentin microhardness and tubule density. Endod Dent Traumatol. 1985; 1:176–179.
Article27. Herrera DR, Santos ZT, Tay LY, Silva EJ, Loguercio AD, Gomes BP. Efficacy of different final irrigant activation protocols on smear layer removal by EDTA and citric acid. Microsc Res Tech. 2013; 76:364–369.
Article28. Cruz-Filho AM, Sousa-Neto MD, Savioli RN, Silva RG, Vansan LP, Pécora JD. Effect of chelating solutions on the microhardness of root canal lumen dentin. J Endod. 2011; 37:358–362.
Article29. Aranda-Garcia AJ, Kuga MC, Chavéz-Andrade GM, Kalatzis-Sousa NG, Hungaro Duarte MA, Faria G, Reis Só MV, Faria NB Jr. Effect of final irrigation protocols on microhardness and erosion of root canal dentin. Microsc Res Tech. 2013; 76:1079–1083.
Article30. Calt S, Serper A. Time-dependent effects of EDTA on dentin structures. J Endod. 2002; 28:17–19.
Article31. De-Deus G, Paciornik S, Mauricio MH. Evaluation of the effect of EDTA, EDTAC and citric acid on the microhardness of root dentine. Int Endod J. 2006; 39:401–407.
Article32. Hamouda IM. Effects of various beverages on hardness, roughness, and solubility of esthetic restorative materials. J Esthet Restor Dent. 2011; 23:315–322.
Article33. Fernández Bertos M, Simons SJ, Hills CD, Carey PJ. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J Hazard Mater. 2004; 112:193–205.34. Chang SW. Chemical characteristics of mineral trioxide aggregate and its hydration reaction. Restor Dent Endod. 2012; 37:188–193.
Article35. Takahashi K. Microbiological, pathological, inflammatory, immunological and molecular biological aspects of periradicular disease. Int Endod J. 1998; 31:311–325.
Article36. Amaral KF, Rogero MM, Fock RA, Borelli P, Gavini G. Cytotoxicity analysis of EDTA and citric acid applied on murine resident macrophages culture. Int Endod J. 2007; 40:338–343.
Article37. Segura JJ, Calvo JR, Guerrero JM, Sampedro C, Jimenez A, Llamas R. The disodium salt of EDTA inhibits the binding of vasoactive intestinal peptide to macrophage membranes: endodontic implications. J Endod. 1996; 22:337–340.
Article38. US food and drug administration. Alpha Hydroxy Acids. updated 2015 Sep 28. Available from: http://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm107940.htm.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of microhardness and fluoride content of tooth structure by fluoride-containing restorative materials
- The Effect of Hemostatic Solution on Dentin Permeability
- Effect of Dentin Bonding Agent Acidity on Surface Microhardness of Mineral Trioxide Aggregate
- Effect of each light curing units on the microhardness and microleakage of composite resin
- Effect of intracanal medicaments used in endodontic regeneration procedures on microhardness and chemical structure of dentin