Korean J Urol.
2011 Apr;52(4):241-246.
Predictive Characteristics of Malignant Pheochromocytoma
- Affiliations
-
- 1Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. hjahn@amc.seoul.kr
- 2Department of General Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
The prognosis of patients with malignant pheochromocytoma is poor, but the predictive factors are not well understood. We aimed to identify the clinical characteristics predictive of malignancy after initial surgical removal in patients with pheochromocytoma.
MATERIALS AND METHODS
We retrospectively reviewed the records of 152 patients diagnosed with pheochromocytoma, including 5 (3.3%) with metastasis at the time of the initial surgical excision and 12 (7.9%) who developed metastasis during follow-up. To determine the factors predictive of malignancy, we compared clinical, radiographical, and urinary chemical findings between patients with benign and malignant disease. Mean follow-up was 41.5 months (range, 0.9-298 months) after surgery.
RESULTS
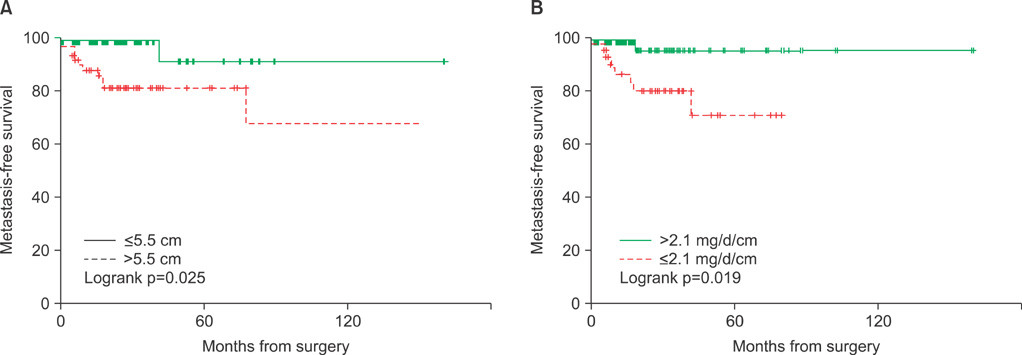
Malignant tumors were significantly larger than benign tumors (11.1+/-4.0 cm vs. 6.2+/-3.4 cm, p<0.001), and postoperative persistence of arterial hypertension was more frequent after removal of malignant than benign tumors (p=0.001). Among the 147 patients without metastatic disease at diagnosis, those who developed metastasis had significantly lower concentrations of urinary catecholamine metabolites per unit of tumor, including vanillylmandelic acid (1.2 vs. 3.7 mg/day/cm, p=0.049), epinephrine (4.5 vs. 168.9 microg/day/cm, p=0.008), and norepinephrine (13.1 vs. 121.8 mg/day/cm, p<0.001). The overall 5-year metastasis-free survival rate was 84.4% and was significantly higher in patients with smaller tumors (< or =5.5 vs. >5.5 cm; 90.6% vs. 81.2%, p=0.025) and higher 24-hour secretion of vanillylmandelic acid (>2.1 vs. < or =2.1 mg/day/cm; 94.9% vs. 70.9%, p=0.019).
CONCLUSIONS
Large tumor size (>5.5 cm) and minimally elevated 24-hour urinary vanillylmandelic acid (< or =2.1 mg/day/cm) were significantly associated with a higher probability of a malignant pheochromocytoma portending a lower metastasis-free survival and mandating more rigorous follow-up after surgery.
MeSH Terms
Figure
Reference
-
1. Guerrero MA, Schreinemakers JM, Vriens MR, Suh I, Hwang J, Shen WT, et al. Clinical spectrum of pheochromocytoma. J Am Coll Surg. 2009. 209:727–732.2. Ram CV, Fierro-Carrion GA. Pheochromocytoma. Semin Nephrol. 1995. 15:126–137.3. Ahlman H. Malignant pheochromocytoma: state of the field with future projections. Ann N Y Acad Sci. 2006. 1073:449–464.4. Bravo EL, Tagle R. Pheochromocytoma: state-of-the-art and future prospects. Endocr Rev. 2003. 24:539–553.5. Adler JT, Meyer-Rochow GY, Chen H, Benn DE, Robinson BG, Sippel RS, et al. Pheochromocytoma: current approaches and future directions. Oncologist. 2008. 13:779–793.6. Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer. 2004. 11:423–436.7. Arnaldi G, Masini AM, Giacchetti G, Taccaliti A, Faloia E, Mantero F. Adrenal incidentaloma. Braz J Med Biol Res. 2000. 33:1177–1189.8. Melicow MM. One hundred cases of pheochromocytoma (107 tumors) at the Columbia-Presbyterian Medical Center, 1926-1976: a clinicopathological analysis. Cancer. 1977. 40:1987–2004.9. Modlin IM, Farndon JR, Shepherd A, Johnston ID, Kennedy TL, Montgomery DA, et al. Phaeochromocytomas in 72 patients: clinical and diagnostic features, treatment and long term results. Br J Surg. 1979. 66:456–465.10. Scott HW Jr, Reynolds V, Green N, Page D, Oates JA, Robertson D, et al. Clinical experience with malignant pheochromocytomas. Surg Gynecol Obstet. 1982. 154:801–818.11. Tischler AS. Pheochromocytoma and extra-adrenal paraganglioma: updates. Arch Pathol Lab Med. 2008. 132:1272–1284.12. John H, Ziegler WH, Hauri D, Jaeger P. Pheochromocytomas: can malignant potential be predicted? Urology. 1999. 53:679–683.13. Guo JZ, Gong LS, Chen SX, Luo BY, Xu MY. Malignant pheochromocytoma: diagnosis and treatment in fifteen cases. J Hypertens. 1989. 7:261–266.14. Mahoney EM, Harrison JH. Malignant pheochromocytoma: clinical course and treatment. J Urol. 1977. 118:225–229.15. Mornex R, Badet C, Peyrin L. Malignant pheochromocytoma: a series of 14 cases observed between 1966 and 1990. J Endocrinol Invest. 1992. 15:643–649.16. Proye C, Vix M, Goropoulos A, Kerlo P, Lecomte-Houcke M. High incidence of malignant pheochromocytoma in a surgical unit. 26 cases out of 100 patients operated from 1971 to 1991. J Endocrinol Invest. 1992. 15:651–663.17. Schlumberger M, Gicquel C, Lumbroso J, Tenenbaum F, Comoy E, Bosq J, et al. Malignant pheochromocytoma: clinical, biological, histologic and therapeutic data in a series of 20 patients with distant metastases. J Endocrinol Invest. 1992. 15:631–642.18. Yoshida S, Hatori M, Noshiro T, Kimura N, Kokubun S. Twenty-six-years' survival with multiple bone metastasis of malignant pheochromocytoma. Arch Orthop Trauma Surg. 2001. 121:598–600.19. Linnoila RI, Keiser HR, Steinberg SM, Lack EE. Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol. 1990. 21:1168–1180.20. Bautista CV, Felis CP, Espinet JM, García JB, Salas JV. Telomerase activity is a prognostic factor for recurrence and survival in rectal cancer. Dis Colon Rectum. 2007. 50:611–620.21. Salmenkivi K, Haglund C, Ristimäki A, Arola J, Heikkilä P. Increased expression of cyclooxygenase-2 in malignant pheochromocytomas. J Clin Endocrinol Metab. 2001. 86:5615–5619.22. Favier J, Plouin PF, Corvol P, Gasc JM. Angiogenesis and vascular architecture in pheochromocytomas: distinctive traits in malignant tumors. Am J Pathol. 2002. 161:1235–1246.23. Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002. 26:551–566.24. Amar L, Servais A, Gimenez-Roqueplo AP, Zinzindohoue F, Chatellier G, Plouin PF. Year of diagnosis, features at presentation, and risk of recurrence in patients with pheochromocytoma or secreting paraganglioma. J Clin Endocrinol Metab. 2005. 90:2110–2116.25. Plouin PF, Chatellier G, Fofol I, Corvol P. Tumor recurrence and hypertension persistence after successful pheochromocytoma operation. Hypertension. 1997. 29:1133–1139.26. Ito Y, Obara T, Yamashita T, Kanbe M, Iihara M. Pheochromocytomas: tendency to degenerate and cause paroxysmal hypertension. World J Surg. 1996. 20:923–926.