Korean J Urol.
2009 Jul;50(7):649-655.
Survival Rates and Related Factors in Men with Hormone-Refractory Prostate Cancer
- Affiliations
-
- 1Department of Urology, Pusan National University School of Medicine, Busan, Korea. 2-wan@hanmail.net
Abstract
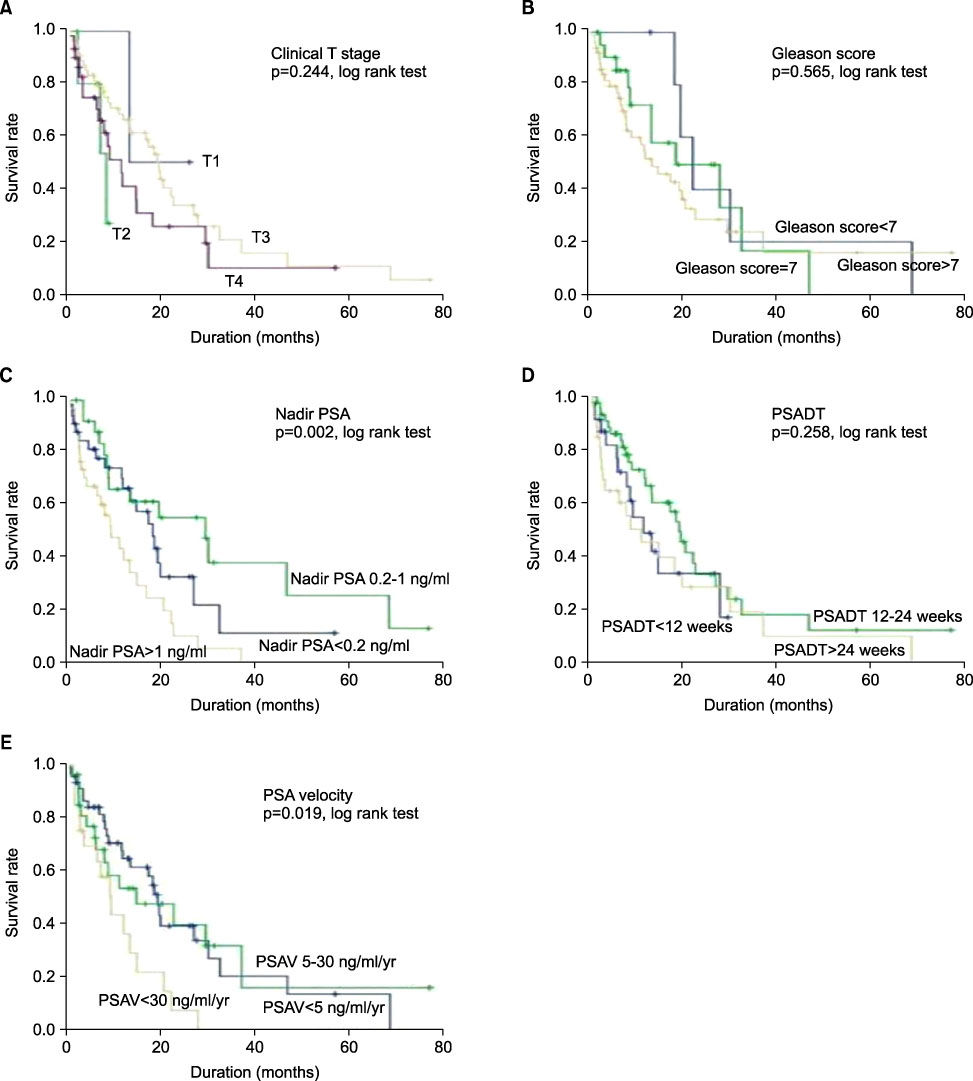
- PURPOSE
We evaluated survival rate in patients with hormone-refractory prostate cancer (HRPC) and the clinical factors that influenced survival rate and time. MATERIALS AND METHODS: The medical records of 96 patients who had HRPC and were not treated with chemotherapy from 2000 to 2008 were reviewed. We evaluated the survival rates at the 1st, 3rd, and 5th year by using Kaplan-Meier survival curves. We also evaluated survival differences according to clinical variables (clinical T stage, Gleason score, nadir prostate-specific antigen [PSA], PSA doubling time, and PSA velocity) by using the log-rank test and the relations between survival rates and these variables by using Cox proportional hazards models. RESULTS: The mean age of the patients was 67.8+/-7.5 years and the mean follow-up period was 23.3+/-13.7 months. Cancer-specific survival rates at the 1st, 3rd, and 5th year were 57.8%, 16.8%, and 10.1%, respectively, and survival differences were significantly related to nadir PSA (p=0.002) and PSA velocity (p=0.019). In the univariate analysis, nadir PSA (p=0.004) and PSA velocity (p=0.024) were related to survival rate, but only nadir PSA remained as a significant variable for survival rate in patients with HRPC in the multivariate analysis (p=0.044). CONCLUSIONS: Cancer-specific survival rates in patients with HRPC at the 1st, 3rd, and 5th year were 57.8%, 16.8%, and 10.1%, respectively, and they were related to nadir PSA. These results may be useful in determining a therapeutic approach in patients with HRPC.
Keyword
MeSH Terms
Figure
Reference
-
1. Korea Central Cancer Registry. Ministry for Health Welfare and Family Affairs. Annual report of the central cancer registry in Korea, National Cancer Center 2003-2005.2. Collette L, de Reijke TM, Schroder FH. Prostate specific antigen: a prognostic marker of survival in good prognosis metastatic prostate cancer? (EORTC 30892). Eur Urol. 2003. 44:182–189.3. Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Urol. 2008. 53:68–80.4. Semeniuk RC, Venner PM, North S. Prostate-specific antigen doubling time is associated with survival in men with hormone-refractory prostate cancer. Urology. 2006. 68:565–569.5. Eisenberger MA. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Chemotherapy for hormone-resistant prostate cancer. Campbell's urology. 1988. 7th ed. Philadelphia: Saunders;2648–2658.6. Oefelein MG, Agarwal PK, Resnick MI. Survival of patients with hormone refractory prostate cancer in the prostate specific antigen era. J Urol. 2004. 171:1525–1528.7. Mike S, Harrison C, Coles B, Staffurth J, Wilt TJ, Mason MD. Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev. 2006. 4:CD005247.8. Smaletz O, Scher HI, Small EJ, Verbel DA, McMillan A, Regan K, et al. Nomogram for overall survival of patients with progressive metastatic prostate cancer after castration. J Clin Oncol. 2002. 20:3972–3982.9. Eisenberger MA, Crawford ED, Wolf M, Blumenstein B, McLeod DG, Benson R, et al. Prognostic factors in stage D2 prostate cancer; important implications for future trials: results of a cooperative intergroup study (INT.0036). The National Cancer Institute Intergroup Study #0036. Semin Oncol. 1994. 21:613–619.10. Matzkin H, Eber P, Todd B, van der Zwaag R, Soloway MS. Prognostic significance of changes in prostate-specific markers after endocrine treatment of stage D2 prostatic cancer. Cancer. 1992. 70:2302–2309.11. Morote J, Esquena S, Abascal JM, Trilla E, Cecchini L, Raventós CX, et al. Usefulness of prostate-specific antigen nadir as predictor of androgen-independent progression of metastatic prostate cancer. Int J Biol Markers. 2005. 20:209–216.12. Kwak C, Jeong SJ, Lee C, Jin RJ, Park TH, Lee SE. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer patients. Korean J Urol. 2001. 42:948–953.13. Park BJ, Lee YG, Ahn HK. Prognostic significance of prostate-specific antigen level two months after maximal androgen blockade in metastatic prostate cancer. Korean J Urol. 2003. 44:855–860.14. Kim KH, Seo YJ, Lee KS. The factors affecting prognosis in patients with metastatic prostate cancer after hormonal therapy. Korean J Urol. 2004. 45:24–28.15. Rozhansky F, Chen MH, Cox MC, Dahut W, Figg WD, D'Amico AV. Prostate-specific antigen velocity and survival for patients with hormone-refractory metastatic prostate carcinoma. Cancer. 2006. 106:63–67.16. D'Amico AV, Renshaw AA, Sussman B, Chen MH. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. JAMA. 2005. 294:440–447.17. Robinson D, Sandblom G, Johansson R, Garmo H, Aus G, Hedlund PO, et al. PSA kinetics provide improved prediction of survival in metastatic hormone-refractory prostate cancer. Urology. 2008. 72:903–907.18. Oudard S, Banu E, Scotte F, Banu A, Medioni J, Beuzeboc P, et al. Prostate-specific antigen doubling time before onset of chemotherapy as a predictor of survival for hormone-refractory prostate cancer patients. Ann Oncol. 2007. 18:1828–1833.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Racial Differences in Prostate Cancer Characteristics and Cancer-Specific Mortality: An Overview
- What's New in Hormone-refractory Prostate Cancer Treatment
- Dramatic Decline of PSA and Symptom Improvement after Estramustine Withdrawal in a Hormone-refractory Prostate Cancer Patient
- Changes in Prostate Cancer Pattern according to Prostate-Specific Antigen Screening Test
- A Continuous Increase in Prevalence of state Cancer in Korea and Its Causes