Obstet Gynecol Sci.
2014 Nov;57(6):457-463. 10.5468/ogs.2014.57.6.457.
Thrombocytosis as a prognostic marker in stage III and IV serous ovarian cancer
- Affiliations
-
- 1Department of Medical Oncology, University Hospital of Lausanne, Lausanne, Switzerland. ivoutsadakis@yahoo.com
- KMID: 2314011
- DOI: http://doi.org/10.5468/ogs.2014.57.6.457
Abstract
OBJECTIVE
Thrombocytosis is an adverse prognostic factor in many types of cancer. We investigated if pre-treatment increased platelet counts provide prognostic information specifically in patients with stage III and IV serous ovarian cancer which is the most common clinical presentation of ovarian cancer.
METHODS
Platelet number on diagnosis of stage III and IV serous ovarian adenocarcinoma was evaluated in 91 patients for whom there were complete follow-up data on progression and survival. Survival and progression free survival of patients with normal platelet counts (150-350 x10(9)/L) was compared with that of patients with thrombocytosis (>350x10(9)/L) by chi2 and logrank tests.
RESULTS
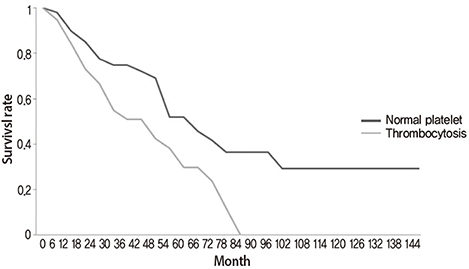
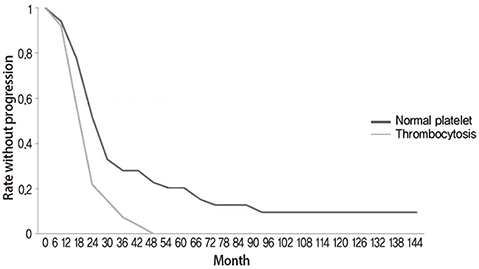
The median age of the patients was 66 years-old. From the 91 patients, 52 (57.1%) had normal platelet counts (median, 273x10(9)/L; range, 153-350) at diagnosis of their disease and 39 patients (42.9%) had thrombocytosis (median, 463x10(9)/L; range, 354-631). In the group of patients with normal platelet counts, 24 of the 52 patients had died with a median survival of 43 months (range, 3-100). In the group of patients with thrombocytosis, 24 of the 39 patients had died with a median survival of 23 months (range, 4-79). In the entire group of 91 patients there was a statistically significant difference of the overall survival and progression-free survival between the two groups (logrank test P=0.02 and P=0.007, respectively).
CONCLUSION
In this retrospective analysis of stage III and IV ovarian cancer patients, thrombocytosis at the time of diagnosis had prognostic value regarding overall survival and progression-free survival.
MeSH Terms
Figure
Cited by 1 articles
-
Association of pretreatment thrombocytosis with prognosis in ovarian cancer: a systematic review and meta-analysis
Qingjian Ye, Juan Cheng, Minjuan Ye, Dong Liu, Yu Zhang
J Gynecol Oncol. 2019;30(1):. doi: 10.3802/jgo.2019.30.e5.
Reference
-
1. Bleeker JS, Hogan WJ. Thrombocytosis: diagnostic evaluation, thrombotic risk stratification, and risk-based management strategies. Thrombosis. 2011; 2011:536062.2. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. 2011; 61:183–203.3. Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008; 109:370–376.4. Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007; 25:2873–2883.5. Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012; 120:612–618.6. Mackay HJ, Brady MF, Oza AM, Reuss A, Pujade-Lauraine E, Swart AM, et al. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int J Gynecol Cancer. 2010; 20:945–952.7. Karabuk E, Kose MF, Hizli D, Taskin S, Karadag B, Turan T, et al. Comparison of advanced stage mucinous epithelial ovarian cancer and serous epithelial ovarian cancer with regard to chemosensitivity and survival outcome: a matched case-control study. J Gynecol Oncol. 2013; 24:160–166.8. Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012; 366:610–618.9. Dawson B, Trapp RG, Dawson-Saunders B. Basic & clinical biostatistics. Norwalk: Appleton & Lange;1994.10. Lawless JF. Statistical models and methods for lifetime data. New York: Wiley;1982.11. Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964; 114:497–500.12. Taucher S, Salat A, Gnant M, Kwasny W, Mlineritsch B, Menzel RC, et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb Haemost. 2003; 89:1098–1106.13. Stravodimou A, Voutsadakis IA. Pretreatment thrombocytosis as a prognostic factor in metastatic breast cancer. Int J Breast Cancer. 2013; 2013:289563.14. Shimada H, Oohira G, Okazumi S, Matsubara H, Nabeya Y, Hayashi H, et al. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surg. 2004; 198:737–741.15. Hwang SG, Kim KM, Cheong JH, Kim HI, An JY, Hyung WJ, et al. Impact of pretreatment thrombocytosis on blood-borne metastasis and prognosis of gastric cancer. Eur J Surg Oncol. 2012; 38:562–567.16. Brown KM, Domin C, Aranha GV, Yong S, Shoup M. Increased preoperative platelet count is associated with decreased survival after resection for adenocarcinoma of the pancreas. Am J Surg. 2005; 189:278–282.17. Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA. Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma. BJU Int. 2000; 86:203–207.18. Hernandez E, Lavine M, Dunton CJ, Gracely E, Parker J. Poor prognosis associated with thrombocytosis in patients with cervical cancer. Cancer. 1992; 69:2975–2977.19. Gungor T, Kanat-Pektas M, Sucak A, Mollamahmutoglu L. The role of thrombocytosis in prognostic evaluation of epithelial ovarian tumors. Arch Gynecol Obstet. 2009; 279:53–56.20. Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012; 38:651–657.21. Li AJ, Madden AC, Cass I, Leuchter RS, Lagasse LD, Karlan BY. The prognostic significance of thrombocytosis in epithelial ovarian carcinoma. Gynecol Oncol. 2004; 92:211–214.22. Allensworth SK, Langstraat CL, Martin JR, Lemens MA, McGree ME, Weaver AL, et al. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol Oncol. 2013; 130:499–504.23. Mermer T, Terek MC, Zeybek B, Ergenoglu AM, Yeniel AO, Ozsaran A, et al. Thrombopoietin: a novel candidate tumor marker for the diagnosis of ovarian cancer. J Gynecol Oncol. 2012; 23:86–90.24. Paule B, Belot J, Rudant C, Coulombel C, Abbou CC. The importance of IL-6 protein expression in primary human renal cell carcinoma: an immunohistochemical study. J Clin Pathol. 2000; 53:388–390.25. Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res. 2000; 6:2702–2706.26. Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002; 2:311–315.27. Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012; 130:2747–2760.28. Ho-Tin-Noe B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011; 9:Suppl 1. 56–65.29. Ho-Tin-Noe B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol. 2009; 175:1699–1708.30. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011; 11:123–134.31. Battinelli EM, Markens BA, Italiano JE Jr. Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011; 118:1359–1369.32. Gunsilius E, Petzer A, Stockhammer G, Nussbaumer W, Schumacher P, Clausen J, et al. Thrombocytes are the major source for soluble vascular endothelial growth factor in peripheral blood. Oncology. 2000; 58:169–174.33. Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011; 20:576–590.34. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009; 119:1420–1428.35. Niers TM, Richel DJ, Meijers JC, Schlingemann RO. Vascular endothelial growth factor in the circulation in cancer patients may not be a relevant biomarker. PLoS One. 2011; 6:e19873.36. Heng DY, Mackenzie MJ, Vaishampayan UN, Bjarnason GA, Knox JJ, Tan MH, et al. Primary anti-vascular endothelial growth factor (VEGF)-refractory metastatic renal cell carcinoma: clinical characteristics, risk factors, and subsequent therapy. Ann Oncol. 2012; 23:1549–1555.37. Sato S, Itamochi H. Bevacizumab and ovarian cancer. Curr Opin Obstet Gynecol. 2012; 24:8–13.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Significance of Thrombocytosis as a Prognostic Factor in Patient with Epithelial Ovarian Cancer
- Prognostic Significance of Thrombocytosis in Gastric Cancer Patients
- Preoperative thrombocytosis predicts prognosis in stage II colorectal cancer patients
- Tumor Angiogenesis and Stage in Ovarian Carcinoma
- The Clinical Significance of Thrombocytosis in Patients presenting with a Pelvic Mass