Obstet Gynecol Sci.
2013 May;56(3):182-189.
Clinical outcomes of vitrified-thawed embryo transfer using a pull and cut straw method
- Affiliations
-
- 1Department of Animal Sciences, Chungbuk National University, Cheongju, Korea. nhkim@chungbuk.ac.kr
- 2Min Byeong Yeol Obstetrics and Gynecology, Cheongju, Korea.
- 3Chungbuk National University School of Medicine, Cheongju, Korea.
- 4Department of Animal Science and Biotechnology, Sangji Youngseo College, Wonju, Korea.
Abstract
OBJECTIVE
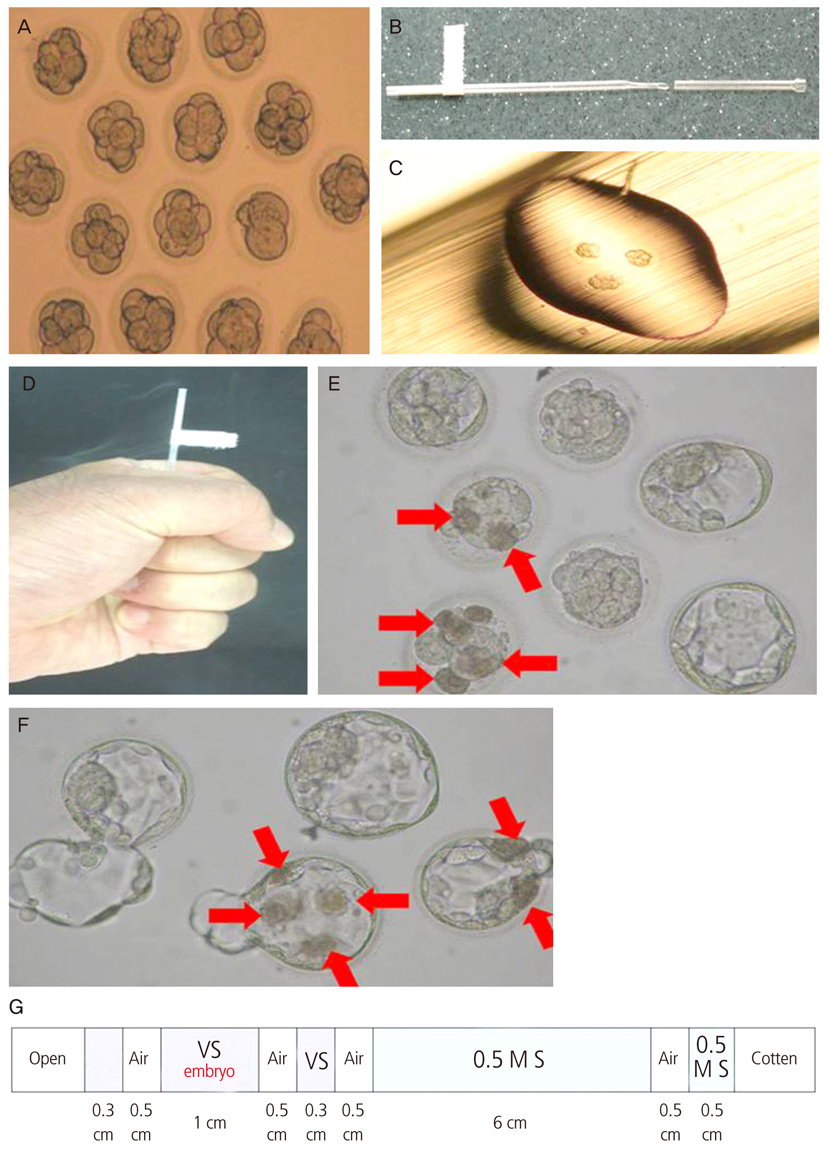
To compare the clinical outcomes of patients with vitrified-thawed embryos transferred using either the 0.25 mL straw method and the pull and cut straw (PNC) method. To evaluate the clinical outcomes of patients with transferred embryos that underwent assisted hatching at the cleaved embryo (day 3) or the blastocyst (day 5) stage.
METHODS
The study population consisted of women who underwent vitrified-warmed embryo transfer between May 2000 and December 2011 and assisted hatching was performed after warming of embryos. Cycles of thawing between assisted hatching treated and non treated groups were compared for survival and pregnancy rates.
RESULTS
The PNC vitrification method improved survival and pregnancy rates in partial lysed embryos. While assisted hatching did not affect the developmental and clinical pregnancy rates of the vitrified-warmed blastocyst group, it did increase the pregnancy rate of poor quality vitrified-warmed cleaved embryos.
CONCLUSION
These results suggest that PNC may increase the number of clinical pregnancies via the vitrification of both cleaved embryos and blastocysts. In addition, selective assisted hatching treatment of embryos that show a poor prognosis after warming may increase the rate of clinical pregnancy.
MeSH Terms
Figure
Reference
-
1. Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985. 313:573–575.2. Desai N, Blackmon H, Szeptycki J, Goldfarb J. Cryoloop vitrification of human day 3 cleavage-stage embryos: post-vitrification development, pregnancy outcomes and live births. Reprod Biomed Online. 2007. 14:208–213.3. Kim HJ, Kim CH, Lee JY, Kwon JH, Hwang D, Kim KC. Effect of cryopreservation day on pregnancy outcomes in frozen-thawed blastocyst transfer. Korean J Reprod Med. 2010. 37:57–64.4. Shin MR, Choi HW, Kim MK, Lee SH, Lee HS, Lim CK. In vitro development and gene expression of frozen-thawed 8-cell stage mouse embryos following slow freezing or vitrification. Clin Exp Reprod Med. 2011. 38:203–209.5. Drobnis EZ, Andrew JB, Katz DF. Biophysical properties of the zona pellucida measured by capillary suction: is zona hardening a mechanical phenomenon? J Exp Zool. 1988. 245:206–219.6. Cohen J, Alikani M, Trowbridge J, Rosenwaks Z. Implantation enhancement by selective assisted hatching using zona drilling of human embryos with poor prognosis. Hum Reprod. 1992. 7:685–691.7. De Vos A, Van Steirteghem A. Zona hardening, zona drilling and assisted hatching: new achievements in assisted reproduction. Cells Tissues Organs. 2000. 166:220–227.8. El-Toukhy T, Khalaf Y, Al-Darazi K, Andritsos V, Taylor A, Braude P. Effect of blastomere loss on the outcome of frozen embryo replacement cycles. Fertil Steril. 2003. 79:1106–1111.9. Tucker MJ, Cohen J, Massey JB, Mayer MP, Wiker SR, Wright G. Partial dissection of the zona pellucida of frozen-thawed human embryos may enhance blastocyst hatching, implantation, and pregnancy rates. Am J Obstet Gynecol. 1991. 165:341–344. discussion 4-5.10. Check JH, Hoover L, Nazari A, O'Shaughnessy A, Summers D. The effect of assisted hatching on pregnancy rates after frozen embryo transfer. Fertil Steril. 1996. 65:254–257.11. Nagy ZP, Taylor T, Elliott T, Massey JB, Kort HI, Shapiro DB. Removal of lysed blastomeres from frozen-thawed embryos improves implantation and pregnancy rates in frozen embryo transfer cycles. Fertil Steril. 2005. 84:1606–1612.12. Martins WP, Rocha IA, Ferriani RA, Nastri CO. Assisted hatching of human embryos: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2011. 17:438–453.13. Schiewe MC, Hazeleger NL, Sclimenti C, Balmaceda JP. Physiological characterization of blastocyst hatching mechanisms by use of a mouse antihatching model. Fertil Steril. 1995. 63:288–294.14. Lanzendorf SE, Nehchiri F, Mayer JF, Oehninger S, Muasher SJ. A prospective, randomized, double-blind study for the evaluation of assisted hatching in patients with advanced maternal age. Hum Reprod. 1998. 13:409–413.15. Vanderzwalmen P, Bertin G, Debauche C, Standaert V, Bollen N, van Roosendaal E, et al. Vitrification of human blastocysts with the Hemi-Straw carrier: application of assisted hatching after thawing. Hum Reprod. 2003. 18:1504–1511.16. Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005. 11:300–308.17. Mukaida T, Nakamura S, Tomiyama T, Wada S, Oka C, Kasai M, et al. Vitrification of human blastocysts using cryoloops: clinical outcome of 223 cycles. Hum Reprod. 2003. 18:384–391.18. Huisman GJ, Fauser BC, Eijkemans MJ, Pieters MH. Implantation rates after in vitro fertilization and transfer of a maximum of two embryos that have undergone three to five days of culture. Fertil Steril. 2000. 73:117–122.19. Carroll J, Depypere H, Matthews CD. Freeze-thaw-induced changes of the zona pellucida explains decreased rates of fertilization in frozen-thawed mouse oocytes. J Reprod Fertil. 1990. 90:547–553.20. Liu HC, Cohen J, Alikani M, Noyes N, Rosenwaks Z. Assisted hatching facilitates earlier implantation. Fertil Steril. 1993. 60:871–875.21. Malter HE, Cohen J. Blastocyst formation and hatching in vitro following zona drilling of mouse and human embryos. Gamete Res. 1989. 24:67–80.22. Hershlag A, Feng HL. Effect of prefreeze assisted hatching on postthaw survival of mouse embryos. Fertil Steril. 2005. 84:1752–1754.23. Cohen J, Inge KL, Suzman M, Wiker SR, Wright G. Videocinematography of fresh and cryopreserved embryos: a retrospective analysis of embryonic morphology and implantation. Fertil Steril. 1989. 51:820–827.24. Cohen J. Assisted hatching of human embryos. J In Vitro Fert Embryo Transf. 1991. 8:179–190.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Associations of post-warming embryo or blastocyst development with clinical pregnancy in vitrified embryo or blastocyst transfer cycles
- Effect of Cryopreservation Day on Pregnancy Outcomes in Frozen-thawed Blastocyst Transfer
- Clinical Significance of Endometrial Thickness and Pattern in Ovum Donation and Cryopreserved - Thawed Embryo Transfer Program
- A retrospective study of single frozen-thawed blastocyst transfer
- The pregnancy outcomes of day-5 poor-quality and day-6 high-quality blastocysts in single blastocyst transfer cycles