Nutr Res Pract.
2015 Feb;9(1):43-48. 10.4162/nrp.2015.9.1.43.
Regular moderate exercise training can alter the urinary excretion of thiamin and riboflavin
- Affiliations
-
- 1Department of Food & Nutrition, Duksung Women's University, 33, Samyangro 114 gil, Dobong-gu, Seoul, 132-714, Korea. yunokcho@duksung.ac.kr
- KMID: 2313808
- DOI: http://doi.org/10.4162/nrp.2015.9.1.43
Abstract
- BACKGROUND/OBJECTIVES
Physical exercise promotes energy producing pathways requiring thiamin and riboflavin as a coenzyme. Therefore, this study investigated the effects of regular exercise training on urinary excretion of thiamin and riboflavin.
MATERIALS/METHODS
Fifty rats were randomly assigned to one of two groups: non-exercise training (NT, n = 25) and regular exercise training (ET, n = 25) for 5 weeks. The rats performed moderate exercise on a treadmill (0.5-0.8 km/hour) for 30 min/day, 5 days/week. Twenty-four hour urine samples were collected at the end of the 0 week, 3rd week, and 5th week of training and thiamin and riboflavin were analyzed.
RESULTS
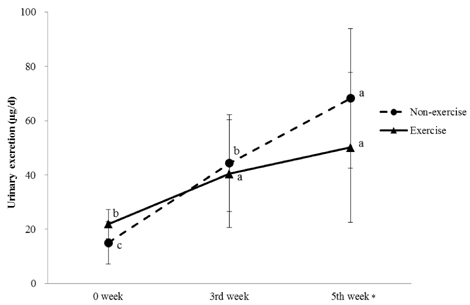
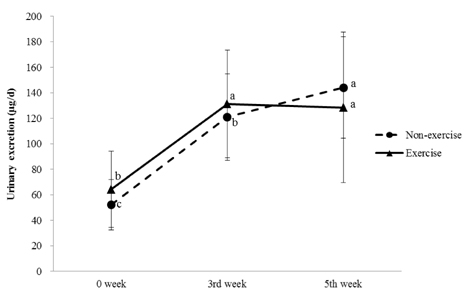
No significant differences in thiamin and riboflavin intakes for each week were observed between the NT and ET groups. Urinary thiamin excretion of each group was the highest at the 5th week compared to the levels at 0 and 3rd week. Urinary thiamin at the 5th week was significantly lower in the ET group than in the NT group. Urinary riboflavin excretion was increased by training duration, however, no difference was observed between NT and ET for each week. At 0 and 3rd week, no significant relationships were observed between dietary intake and urinary excretion of thiamin and riboflavin, however, at the 5th week, urinary excretion was significantly increased by dietary intake only in the NT group (P < 0.05). Thiamin excretion of both NT and ET groups was significantly increased with riboflavin excretion at the 5th week (P < 0.01).
CONCLUSION
Regular moderate exercise training increased urinary excretion of thiamin. Dietary intakes and urinary excretions of thiamin and riboflavin showed positive correlation in both the exercise training and non-exercise training groups as the exercise training period went by, while the correlations in the exercise training group were weaker than those in the non-exercise training group. Therefore, regular exercise training can alter the urinary excretion of thiamin and riboflavin in rats.
Keyword
Figure
Cited by 1 articles
-
The effects of exercise training and acute exercise duration on plasma folate and vitamin B12
Young-Nam Kim, Ji Hyeon Hwang, Youn-Ok Cho
Nutr Res Pract. 2016;10(2):161-166. doi: 10.4162/nrp.2016.10.2.161.
Reference
-
1. Williams MH. Dietary supplements and sports performance: introduction and vitamins. J Int Soc Sports Nutr. 2004; 1:1–6.
Article2. McCormick DB. Coenzymes, biochemistry. In : Dulbecco R, editor. Encyclopedia of Human Biology. 2nd ed. San Diego (CA): Academic Press;1997. p. 847–864.3. Woolf K, Manore MM. B-vitamins and exercise: does exercise alter requirements? Int J Sport Nutr Exerc Metab. 2006; 16:453–484.
Article4. Johnson Gubler C. Thiamin. In : Machlin LJ, editor. Handbook of Vitamins: Nutritional, Biochemical, and Clinical Aspects. New York (NY): Marcel Dekker;1984. p. 245–295.5. Al-Tamimi EK, Haub MD. Thiamin. In : Driskell JA, Wolinsky I, editors. Sports Nutrition: Vitamins and Trace Elements. 2nd ed. Boca Raton (FL): CRC Press;2006. p. 47–60.6. Manore MM. Effect of physical activity on thiamine, riboflavin, and vitamin B-6 requirements. Am J Clin Nutr. 2000; 72:598S–606S.
Article7. Kreider RB, Wilborn CD, Taylor L, Campbell B, Almada AL, Collins R, Cooke M, Earnest CP, Greenwood M, Kalman DS, Kerksick CM, Kleiner SM, Leutholtz B, Lopez H, Lowery LM, Mendel R, Smith A, Spano M, Wildman R, Willoughby DS, Ziegenfuss TN, Antonio J. ISSN exercise & sport nutrition review: research & recommendations. J Int Soc Sports Nutr. 2010; 7:7.8. Ravi Kiran T, Subramanyam MV, Asha Devi S. Swim exercise training and adaptations in the antioxidant defense system of myocardium of old rats: relationship to swim intensity and duration. Comp Biochem Physiol B Biochem Mol Biol. 2004; 137:187–196.
Article9. Lira FS, Koyama CH, Yamashita AS, Rosa JC, Zanchi NE, Batista ML Jr, Seelaender MC. Chronic exercise decreases cytokine production in healthy rat skeletal muscle. Cell Biochem Funct. 2009; 27:458–461.
Article10. Campbell PT, Gross MD, Potter JD, Schmitz KH, Duggan C, McTiernan A, Ulrich CM. Effect of exercise on oxidative stress: a 12-month randomized, controlled trial. Med Sci Sports Exerc. 2010; 42:1448–1453.11. Choi EY, Cho YO. The influence of different durations of aerobic exercise on fuel utilization, lactate level and antioxidant defense system in trained rats. Nutr Res Pract. 2014; 8:27–32.
Article12. Losa R, Sierra MI, Fernández A, Blanco D, Buesa JM. Determination of thiamine and its phosphorylated forms in human plasma, erythrocytes and urine by HPLC and fluorescence detection: a preliminary study on cancer patients. J Pharm Biomed Anal. 2005; 37:1025–1029.
Article13. Gatautis VJ, Naito HK. Liquid-chromatographic determination of urinary riboflavin. Clin Chem. 1981; 27:1672–1675.
Article14. Zempleni J, Galloway JR, McCormick DB. Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans. Am J Clin Nutr. 1996; 63:54–66.
Article15. Horwitt MK. Interpretations of requirements for thiamin, riboflavin, niacin-tryptophan, and vitamin E plus comments on balance studies and vitamin B-6. Am J Clin Nutr. 1986; 44:973–985.
Article16. Powers HJ. Current knowledge concerning optimum nutritional status of riboflavin, niacin and pyridoxine. Proc Nutr Soc. 1999; 58:435–440.
Article17. Tasevska N, Runswick SA, McTaggart A, Bingham SA. Twenty-four-hour urinary thiamine as a biomarker for the assessment of thiamine intake. Eur J Clin Nutr. 2008; 62:1139–1147.
Article18. Fukuwatari T, Shibata K. Urinary water-soluble vitamins and their metabolite contents as nutritional markers for evaluating vitamin intakes in young Japanese women. J Nutr Sci Vitaminol (Tokyo). 2008; 54:223–229.
Article19. Tsuji T, Fukuwatari T, Sasaki S, Shibata K. Urinary excretion of vitamin B1, B2, B6, niacin, pantothenic acid, folate, and vitamin C correlates with dietary intakes of free-living elderly, female Japanese. Nutr Res. 2010; 30:171–178.
Article20. Fogelholm M. Micronutrient status in females during a 24-week fitness-type exercise program. Ann Nutr Metab. 1992; 36:209–218.
Article21. Choi SK, Baek SH, Choi SW. The effects of endurance training and thiamine supplementation on anti-fatigue during exercise. J Exerc Nutrition Biochem. 2013; 17:189–198.
Article22. Choi EY, Cho YO. Moderate physical training can increase muscle glycogen levels but does not alter protein levels with exercise in rats. Nutr Sci. 2006; 9:112–116.23. Choi EY, Cho YO. The effects of physical training on antioxidative status under exercise-induced oxidative stress. Nutr Res Pract. 2007; 1:14–18.
Article24. Masuda H, Matsumae H, Masuda T, Hatta H. A thiamin derivative inhibits oxidation of exogenous glucose at rest, but not during exercise. J Nutr Sci Vitaminol (Tokyo). 2010; 56:9–12.
Article25. Shibata K, Fukuwatari T, Watanabe T, Nishimuta M. Intra- and inter-individual variations of blood and urinary water-soluble vitamins in Japanese young adults consuming a semi-purified diet for 7 days. J Nutr Sci Vitaminol (Tokyo). 2009; 55:459–470.
Article26. Vallerand AL, Cuerrier JP, Shapcott D, Vallerand RJ, Gardiner PF. Influence of exercise training on tissue chromium concentrations in the rat. Am J Clin Nutr. 1984; 39:402–409.
Article27. Hunter KE, Turkki PR. Effect of exercise on riboflavin status of rats. J Nutr. 1987; 117:298–304.
Article28. Gollnick PD, Armstrong RB, Saltin B, Saubert CW 4th, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973; 34:107–111.
Article29. Molé PA, Oscai LB, Holloszy JO. Adaptation of muscle to exercise. Increase in levels of palmityl CoA synthetase, carnitine palmityltransferase, and palmityl CoA dehydrogenase, and in the capacity to oxidize fatty acids. J Clin Invest. 1971; 50:2323–2330.30. Belko AZ, Obarzanek E, Roach R, Rotter M, Urban G, Weinberg S, Roe DA. Effects of aerobic exercise and weight loss on riboflavin requirements of moderately obese, marginally deficient young women. Am J Clin Nutr. 1984; 40:553–561.
Article31. Belko AZ, Meredith MP, Kalkwarf HJ, Obarzanek E, Weinberg S, Roach R, McKeon G, Roe DA. Effects of exercise on riboflavin requirements: biological validation in weight reducing women. Am J Clin Nutr. 1985; 41:270–277.
Article32. Sato A, Shimoyama Y, Ishikawa T, Murayama N. Dietary thiamin and riboflavin intake and blood thiamin and riboflavin concentrations in college swimmers undergoing intensive training. Int J Sport Nutr Exerc Metab. 2011; 21:195–204.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of exercise training and acute exercise duration on plasma folate and vitamin B12
- Effects of Soy Isoflavones Supplementation and Exercise on Urinary Calcium, Magnecium, Copper and Zinc Excretion in Postmenopausal Women
- Comparison of Riboflavin Status between Traditional Farming Women and Commercial Farming Women in Korea
- Riboflavin and Thiamine Absorption
- Exercise Rehabilitation for Frail Elderly