Nutr Res Pract.
2010 Dec;4(6):486-491.
Hypoglycemic effects of Welsh onion in an animal model of diabetes mellitus
- Affiliations
-
- 1Department of Smart Foods and Drugs, School of Food and Life Science, Institute for Food Sciences, Inje University, 607 Obang-dong, Gimhae 621-749, Korea. fdsnkiji@inje.ac.kr
- 2Department of Nutrition, Pusan Paik Hospital, Busan 633-165, Korea.
- 3Department of Genetic Engineering, Bk 21 Center for Silver-Bio Industrialization Dong-A University, Busan 604-714, Korea.
Abstract
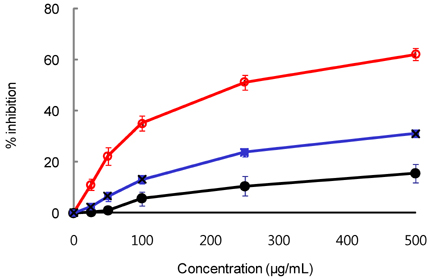
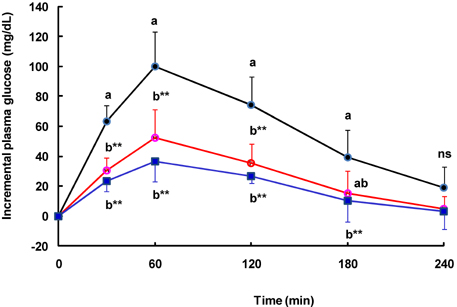
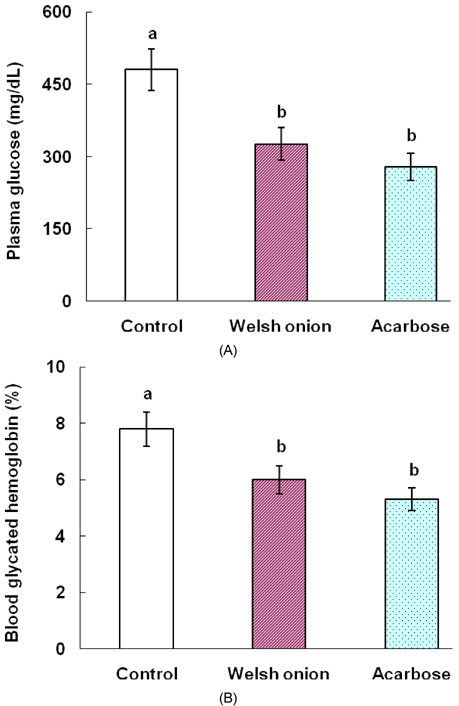
- Tight control of blood glucose is the most important strategy for the treatment of diabetes mellitus. Here, we investigated the beneficial effects of Welsh onion on fasting and postprandial hyperglycemia. Inhibitory activities of hot water extracts from the green stalk and white bulb, which are the edible portions of the Welsh onion, and the fibrous root extract against yeast alpha-glucosidase were measured in vitro. To study the effects of Welsh onion on postprandial hyperglycemia, a starch solution (1 g/kg) with and without Welsh onion fibrous root extract (500 mg/kg) or acarbose (50 mg/kg) was administered to streptozotocin-induced diabetic rats after an overnight fast. Postprandial plasma glucose levels were measured and incremental areas under the response curve were calculated. To study the hypoglycemic effects of chronic feeding of Welsh onion, five-week-old db/db mice were fed an AIN-93G diet or a diet containing either Welsh onion fibrous root extract at 0.5% or acarbose at 0.05% for 7 weeks after 1 week of adaptation. Fasting plasma glucose and blood glycated hemoglobin were measured. Compared to the extract from the edible portions of Welsh onion, the fibrous root extract showed stronger inhibition against yeast alpha-glucosidase, with an IC50 of 239 microg/mL. Oral administration of Welsh onion fibrous root extract (500 mg/kg) and acarbose (50 mg/kg) significantly decreased incremental plasma glucose levels 30-120 min after oral ingestion of starch as well as the area under the postprandial glucose response curve, compared to the control group (P < 0.01). The plasma glucose and blood glycated hemoglobin levels of the Welsh onion group were significantly lower than those of the control group (P < 0.01), and were not significantly different from those fed acarbose. Thus, we conclude that the fibrous root of Welsh onion is effective in controlling hyperglycemia in animal models of diabetes mellitus.
MeSH Terms
-
Acarbose
Administration, Oral
alpha-Glucosidases
Animals
Blood Glucose
Diabetes Mellitus
Diet
Eating
Fasting
Glucose
Hemoglobins
Humans
Hyperglycemia
Hypoglycemic Agents
Inhibitory Concentration 50
Mice
Models, Animal
Onions
Plasma
Rats
Starch
Water
Yeasts
Acarbose
Blood Glucose
Glucose
Hemoglobins
Hypoglycemic Agents
Starch
Water
alpha-Glucosidases
Figure
Reference
-
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998. 21:1414–1431.
Article2. American Diabetes Association (ADA). Summary of revisions for the 2008 clinical practice recommendations. Diabetes Care. 2008. 31:S3–S4.3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.4. Abrahamson MJ. Optimal glycemic control in type 2 diabetes mellitus: fasting and postprandial glucose in context. Arch Intern Med. 2004. 164:486–491.
Article5. Standl E, Baumgartl HJ, Füchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999. 1:215–220.
Article6. Sels JP, Huijberts MS, Wolffenbuttel BH. Miglitol, a new alpha-glucosidase inhibitor. Expert Opin Pharmacother. 1999. 1:149–156.7. Fujita H, Yamagami T, Ohshima K. Fermented soybean-derived water-soluble Touchi extract inhibits alpha-glucosidase and is antiglycemic in rats and humans after single oral treatments. J Nutr. 2001. 131:1211–1213.
Article8. Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, Min BH. Inhibitory effect of aqueous extract from the gall of Rhus Chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol. 2003. 85:283–287.
Article9. Gholamhoseinian A, Fallah H, Sharifi far F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on alpha-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine. 2009. 16:935–941.
Article10. Joo HJ, Kang MJ, Seo TJ, Kim HA, Yoo SJ, Lee SK, Lim HJ, Byun BH, Kim JI. The hypoglycemic effect of Saururus chinensis Baill in animal models of diabetes mellitus. Food Sci Biotechnol. 2006. 15:413–417.11. Wang H, Du YJ, Song HC. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010. 123:6–13.
Article12. Yamamoto Y, Aoyama S, Hamaguchi N, Rhi GS. Antioxidative and antihypertensive effects of Welsh onion on rats fed with a high-fat high-sucrose diet. Biosci Biotechnol Biochem. 2005. 69:1311–1317.
Article13. Yamamoto Y, Yasuoka A. Welsh onion attenuates hyperlipidemia in rats fed on high-fat high-sucrose diet. Biosci Biotechnol Biochem. 2010. 74:402–404.
Article14. Yoshino G, Hirano T, Kazumi T. Dyslipidemia in diabetes mellitus. Diabetes Res Clin Pract. 1996. 33:1–14.
Article15. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003. 17:24–38.
Article16. Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA, Matsuhisa M, Yamasaki Y. Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic β-cell dysfunction and insulin resistance. Int J Biochem Cell Biol. 2005. 37:1595–1608.
Article17. Youn JY, Park HY, Cho KH. Anti-hypergltcemic activity of Commelina communis L.: inhibition of α-glucosidase. Diabetes Res Clin Pract. 2004. 66:S149–S155.18. Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of α-glucosidase inhibitors from Tochu-cha. Biosci Biotechnol Biochem. 1997. 61:177–178.
Article19. Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi Sh, Farhangi A, Allah Verdi A, Mofidian SMA, Lame Rad B. Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem. 2007. 22:60–64.
Article20. Lee SK, Hwang JY, Song JH, Jo JR, Kim MJ, Kim ME, Kim JI. Inhibitory activity of Euonymus alatus against alpha-glucosidase in vitro and in vivo. Nutr Res Pract. 2007. 1:184–188.21. Raabo E, Terkildsen TC. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960. 12:402–407.
Article22. Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993. 123:1939–1951.
Article23. Schifreen RS, Hickingbotham MJ, Bowers GN Jr. Accuracy, precision, and stability in measurement of hemoglobin A1c by "high performance" cation-exchange chromatography. Clin Chem. 1980. 26:466–472.
Article24. Hanefeld M. The role of acarbose in the treatment of non-insulin-dependent diabetes mellitus. J Diabetes Complications. 1998. 12:228–237.
Article25. Functional Food [Internet]. Korea Food and Drug Administration. cited 2010 Aug 30. Available from: http://hfoodi.kfda.go.kr.26. Saito M. Role of FOSHU (food for specified health uses) for healthier life. Yakugaku Zasshi. 2007. 127:407–416.
Article27. Haller H. The clinical importance of postprandial glucose. Diabetes Res Clin Pract. 1998. 40:S43–S49.
Article28. Ceriello A, Davidson J, Hanefeld M, Leiter L, Monnier L, Owens D, Tajima N, Tuomilehto J. International Prandial Glucose Regulation Study Group. Postprandial hyperglycaemia and cardiovascular complications of diabetes: an update. Nutr Metab Cardiovasc Dis. 2006. 16:453–456.
Article29. Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care. 1999. 22:960–964.
Article30. Balfour JA, McTavish D. Acarbose. An update of its pharmacology and therapeutic use in diabetes mellitus. Drugs. 1993. 46:1025–1054.31. Coniff RF, Shapiro JA, Robbins D, Kleinfield R, Seaton TB, Beisswenger P, McGill JB. Reduction of glycosylated hemoglobin and postprandial hyperglycemia by acarbose in patients with NIDDM. A placebo-controlled dose-comparison study. Diabetes Care. 1995. 18:817–824.
Article32. Meneilly GS, Ryan EA, Radziuk J, Lau DC, Yale JF, Morais J, Chiasson JL, Rabasa-Lhoret R, Maheux P, Tessier D, Wolever T, Josse RG, Elahi D. Effect of acarbose on insulin sensitivity in elderly patients with diabetes. Diabetes Care. 2000. 23:1162–1167.
Article33. Lebovitz HE. α-Glucosidase inhibitors as agents in the treatment of diabetes. Diabetes Rev. 1998. 6:132–145.34. Dolan PL, Tapscott EB, Peterson RG, Dohm GL. Effects of feeding acarbose on muscle glucose transport and GLUT4 protein in lean and obese diabetic (ZDFIGmi-fa) rats. J Nutr Biochem. 1997. 8:322–327.
Article35. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. 2002. 48:436–472.
Article36. LeRoith D, Smith DO. Monitoring glycemic control: the cornerstone of diabetes care. Clin Ther. 2005. 27:1489–1499.37. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002. 346:393–403.
Article38. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000. 321:405–412.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Occurrence of Anthracnose on Welsh Onion Caused by Colletotrichum circinans
- Treatment Strategy for Diabetes with Cardiovascular Disease
- Monotherapy in Type 2 Diabetes Mellitus Patients 2017: A Position Statement of the Korean Diabetes Association
- Use of Oral Hypoglycemic Agents in Type 2 Diabetic Patients with Hepatic Dysfunction
- Combination Therapy of Oral Hypoglycemic Agents in Patients with Type 2 Diabetes Mellitus