Lab Med Online.
2015 Jan;5(1):6-14. 10.3343/lmo.2015.5.1.6.
Performance Evaluation of Glucometer Barozen H Based on ISO 15197 Standards
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Sungkyunkwan University School of Medicine, Seoul, Korea. nayadoo@hanmail.net
- 2Department of Laboratory Medicine and Genetics, Samsung Medical Center, Seoul, Korea.
- KMID: 2312261
- DOI: http://doi.org/10.3343/lmo.2015.5.1.6
Abstract
- BACKGROUND
We have evaluated the analytical performance of Barozen H (i-SENS Inc. Seoul, Korea), which was developed for glucose testing and can be connected to hospital information network systems.
METHODS
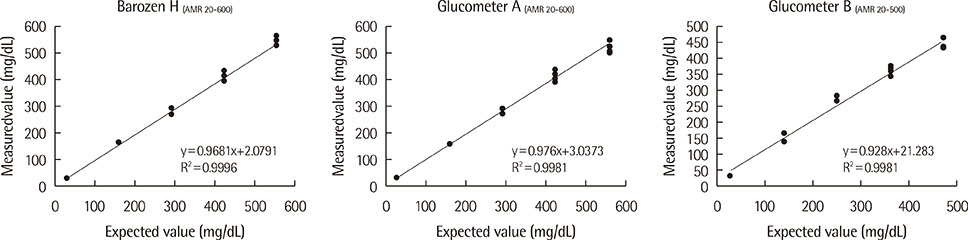
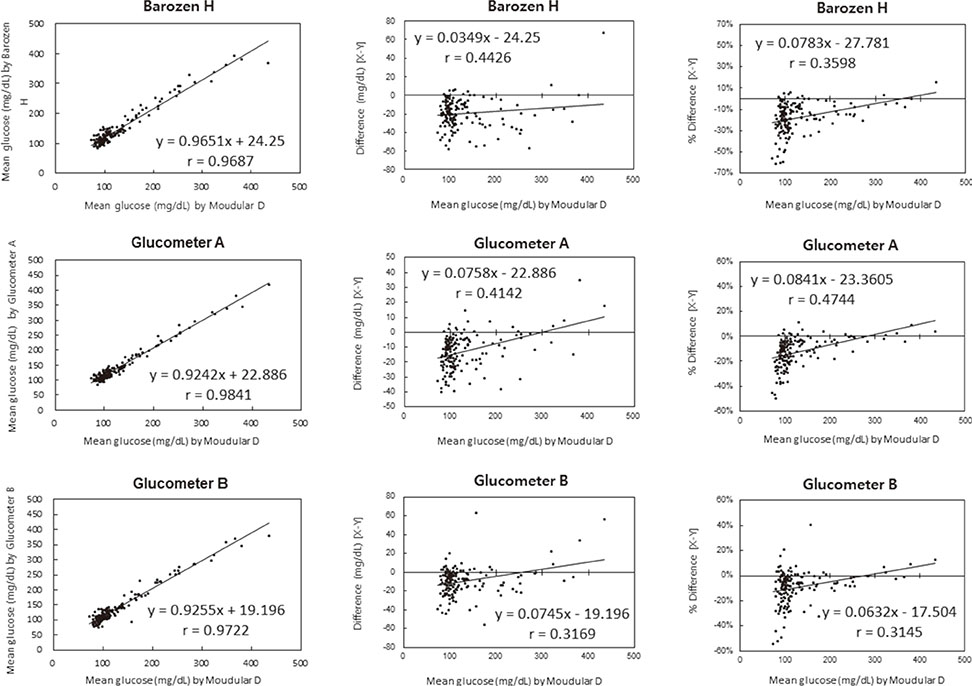
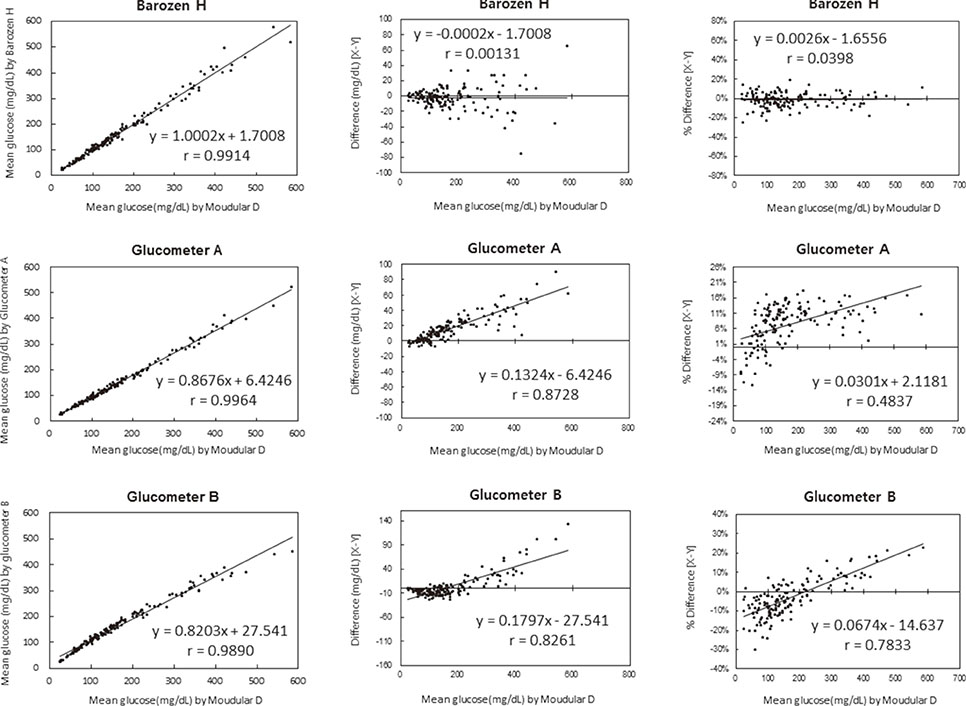
We evaluated the precision of ten glucometers based on repeatability and intermediate precision according to the guidelines of the International Standardization Organization (ISO) 15197:2011. The linearity of patient samples was in the range of 30.7-551.2 mg/dL. The accuracy of the results of Barozen H and the correlation of these results and those of the two other glucometers in comparisons with the Modular D reference device (Roche Diagnostics Ltd., Switzerland) were evaluated using 150 capillary blood and venous whole blood samples.
RESULTS
The ranges for the repeatability and intermediate precision of ten Barozen H glucometers were 1.58-4.61% and 2.85-5.48%, respectively. The linearity was expressed by y=0.9681x+2.0791, and the coefficient of determination (R2) was 0.9996. When venous whole blood samples were used, the correlation coefficient (r) was 0.9914. When glucose levels were under 100 mg/dL, 95.2% of Barozen H results were within +/-15 mg/dL, and when glucose levels were 100 mg/dL or higher, 97.2% were within +/-15%. When capillary blood samples were used, 41.4% (under 100 mg/dL) and 55.4% (100 mg/dL or higher) of Barozen H results were within +/-15 mg/dL and +/-15%, respectively.
CONCLUSIONS
Barozen H provided reliable results and satisfied the ISO15197:2011 criteria when venous whole blood samples were used. It is thought to be clinically useful as a hospital point-of-care glucometer.
Keyword
Figure
Cited by 3 articles
-
Influence of Vitamin C and Maltose on the Accuracy of Three Models of Glucose Meters
Jooyoung Cho, Sunyoung Ahn, Jisook Yim, Younjung Cheon, Seok Hoon Jeong, Sang-Guk Lee, Jeong-Ho Kim
Ann Lab Med. 2016;36(3):271-274. doi: 10.3343/alm.2016.36.3.271.Self-Monitoring Blood Glucose Meter: Is Your Glucose Meter Accurate?
Seon Yeong Park
J Korean Diabetes. 2015;16(1):38-42. doi: 10.4093/jkd.2015.16.1.38.Evaluation of the Self-Testing Blood Glucose Monitoring System GlucoDr.S According to ISO 15197:2013 Guidelines
Namhee Kim, Bo Gyung Kim, Sun-Hee Jun, Kyunghoon Lee, Tae Jung Oh, Sung Hee Choi, Soo Lim, Sang Hoon Song, Woon Heung Song, Junghan Song, Hak Chul Jang
Lab Med Online. 2018;8(3):77-86. doi: 10.3343/lmo.2018.8.3.77.
Reference
-
1. Korea Centers for Disease Control and Prevention. The fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1) 2010. Korea Centers for Disease Control and Prevention;2012.2. Kleppe K. The effect of hydrogen peroxide on glucose oxidase from Aspergillus niger. Biochemistry. 1966; 5(1):139–143.
Article3. Bao J, Furumoto K, Fukunaga K, Nakao K. A kinetic study on air oxidation of glucose catalyzed by immobilized glucose oxidase for production of calcium gluconate. Biochem Eng J. 2001; 8:91–102.
Article4. Mirón J, González MP, Pastrana L, Murado MA. Diauxic production of glucose oxidase by Aspergillus niger in submerged culture a dynamic model. Enz Microb Technol. 2002; 31:615–620.
Article5. Ferri S, Kojima K, Sode K. Review of glucose oxidases and glucose dehydrogenases: a bird's eye view of glucose sensing enzymes. J Diabetes Sci Technol. 2011; 5:1068–1076.
Article6. U.S. Food and Drug Administration. FDA public health notification: Potentially fatal errors with GDH-PQQ glucose monitoring technology. Accessed May 27, 2011. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm.7. The International Organization for Standardization. In vitro diagnostic test systems -Requirements for blood glucose monitoring systems for self-testing in managing diabetes mellitus. ISO/TC 212/SC. Draft International Standard ISO/DIS 15197. Geneva: ISO;2011.8. Khan MI, Weinstock RS. Carbohydrates. In : Mcpherson RA, Pincus MR, editors. Henry's clinical diagnosis and management by laboratory methods. 22nd ed. Philadelphia: WB Saunders;2011. p. 210–225.9. Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; Approved guideline - Second Edition. CLSI document EP09-A2-IR. Wayne, PA: Clinical and Laboratory Standards Institute;2010.10. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: A statistical approach: Approved guideline. NCCLS document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.11. Medicare, Medicaid and CLIA programs; regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA)-HCFA. Final rule with comment period. Fed Regist. 1992; 57:7002–7186.12. International Diabetes Federation. IDF Diabetes Atlas. 5th Edition. Brussels: International Diabetes Federation;2011.13. Kost GJ, Nguyen TH, Tang Z. Whole-blood glucose and lactate. Trilayer biosensors, drug interference, metabolism, and practice guidelines. Arch Pathol Lab Med. 2000; 124:1128–1134.14. Ybarra J, Isern J. Leukocytosis-induced artifactual hypoglycemia. Endocr J. 2003; 50:481–482.
Article15. D'Orazio P, Burnett RW, Fogh-Andersen N, Jacobs E, Kuwa K, Kulpmann WR, et al. Approved IFCC recommendation on reporting results for blood glucose: International Federation of Clinical Chemistry and Laboratory Medicine Scientific Division, Working Group on Selective Electrodes and Point-of-Care Testing (IFCC-SD-WG-SEPOCT). Clin Chem Lab Med. 2006; 44:1486–1490.16. Johnson RN, Baker JR. Error detection and measurement in glucose monitors. Clin Chim Acta. 2001; 307:61–67.
Article17. Burnett RW, D'Orazio P, Fogh-Andersen N, Kuwa K, Kulpmann WR, Larsson L, et al. Approved IFCC recommendation on reporting results for blood glucose. Clin Chim Acta. 2001; 307:205–209.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of BAROZEN H, a Networking Blood Glucose Monitoring System for Medical Institutions
- Analytic Performance Evaluation of Blood Monitoring System G400 according to ISO 15197:2013
- Accuracy of Capillary Blood Glucose Test When Fasting in Diabetes Patients or General Population: Performance Evaluation of G300 Based on ISO 15197:2013 Standards
- Evaluation of the Self-Testing Blood Glucose Monitoring System GlucoDr.S According to ISO 15197:2013 Guidelines
- Performance Evaluation of B. Braun Omnitest 5 Blood Glucose Monitoring System for Self-Monitoring of Blood Glucose