Lab Med Online.
2014 Jan;4(1):8-14.
Mobilization of Peripheral Blood Stem Cells for Autologous Transplantation in Patients with Hematologic Malignancies
- Affiliations
-
- 1Department of Laboratory Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea. sgkim@cu.ac.kr
- 2Division of Hematology-Oncology, Department of Internal Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea.
Abstract
- BACKGROUND
Autologous peripheral blood-stem cell transplantation (autoPBSCT) is the treatment of choice for hematologic malignancy, because the technique requires neither general anesthesia nor surgical intervention, amongst many other advantages. Despite these benefits, the risk of hematologic malignancy, as well as the effect of patient age and sex on the prediction of successful collection of autoPBSCT are still unclear. The purpose of this study was to examine whether the hematologic diagnosis of the disease, and age or sex affect the mobilization of CD34+ cells and mononuclear cells.
METHODS
We retrospectively investigated 30 (6 multiple myeloma, 11 diffuse large B-cell lymphoma, 8 acute myeloid leukemia, 2 acute lymphoid leukemia, and 3 T-cell lymphoma) patients who underwent autoPBSCT between 2008 and 2011 at Daegu Catholic University Hospital.
RESULTS
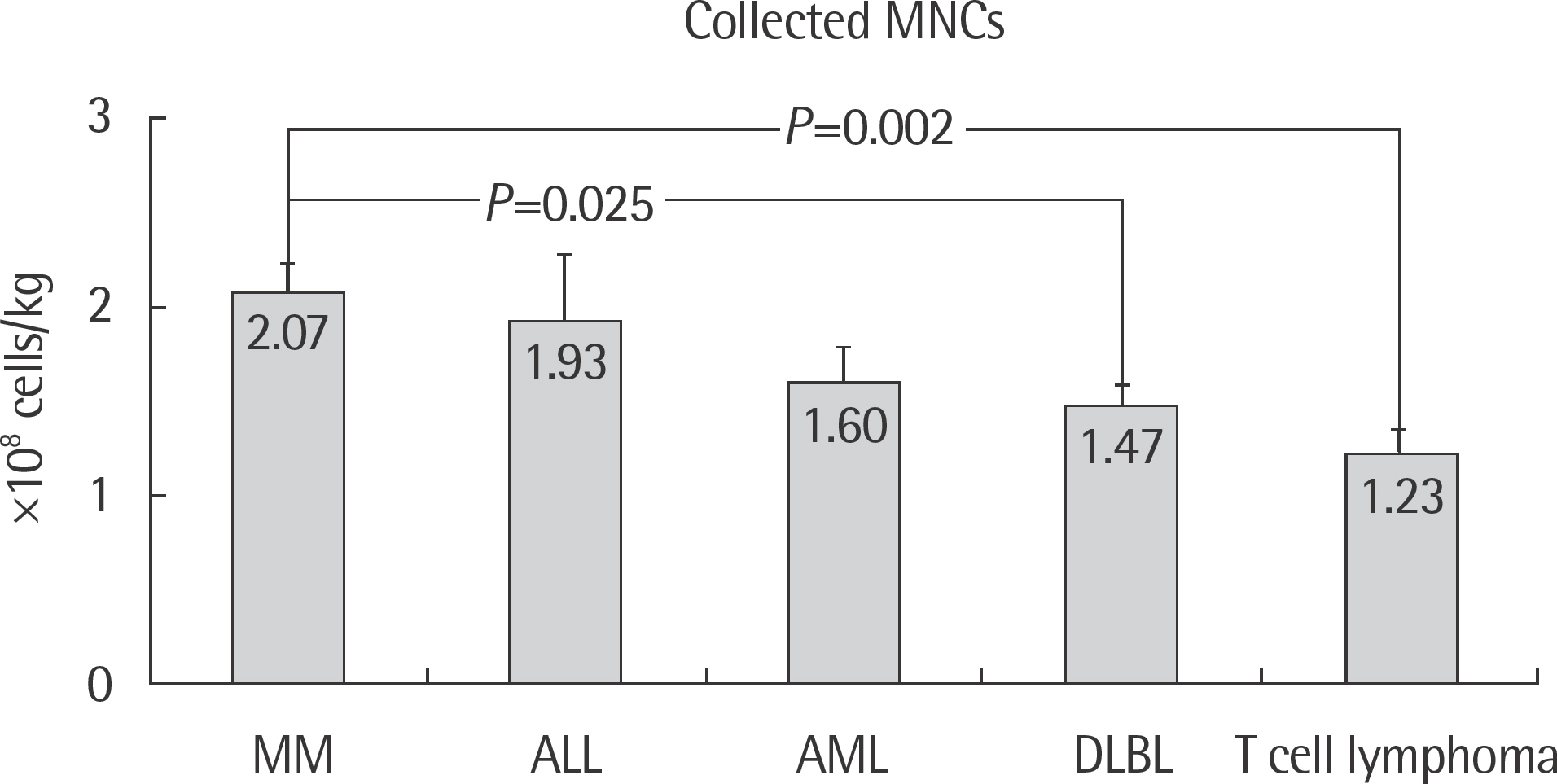
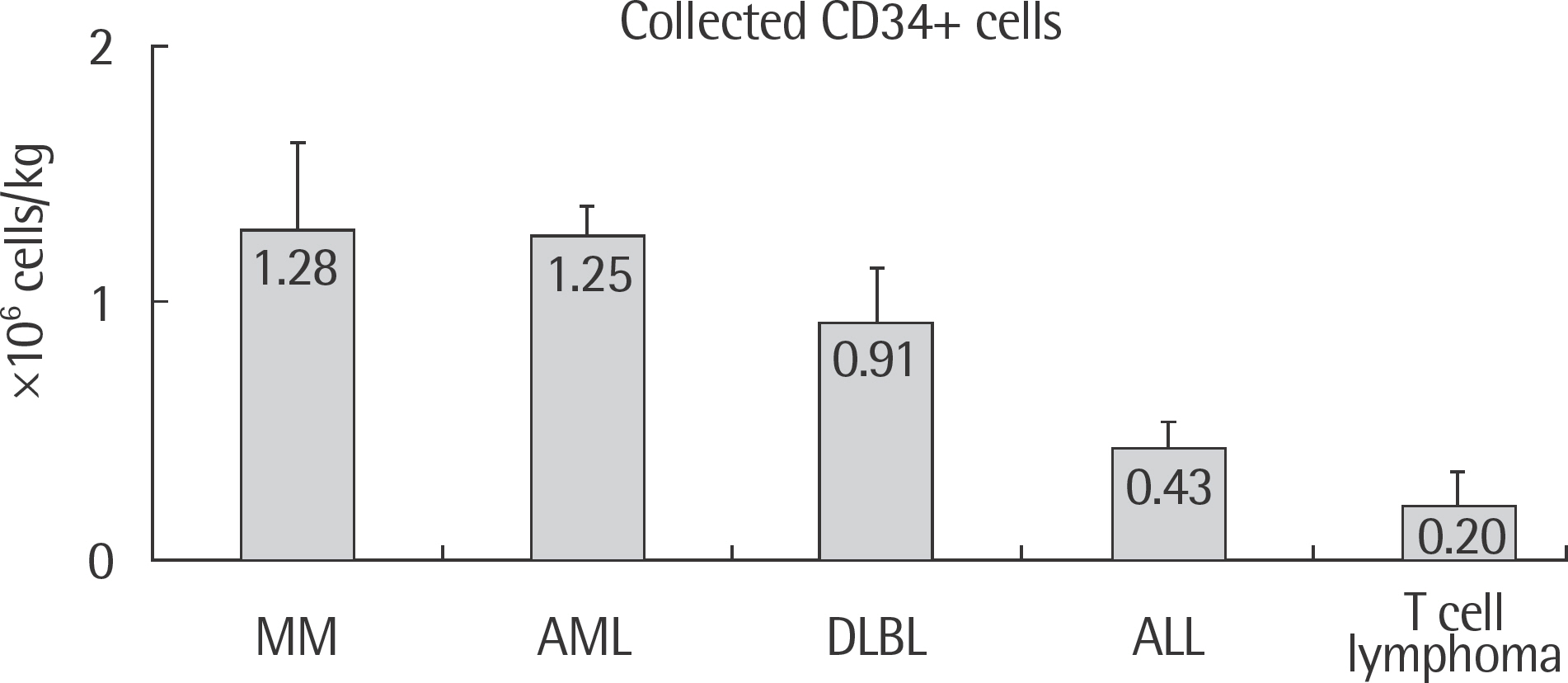
Patients with multiple myeloma had the highest average of both mononuclear cell (MNC) (2.07+/-0.67x10(8) cells/kg) and CD34+ cell (1.28+/-0.58x10(6) cells/kg) counts. Patients with T-cell lymphoma had both the lowest MNC (1.23+/-0.49x10(8) cells/kg) and CD34+ cell (0.20+/-0.6x10(6) cells/kg) counts. Male patients showed greater collected CD34+ cell counts (0.96+/-1.38x10(6) cells/kg) and MNC counts (1.71+/-0.76x10(8) cells/kg) than the female patients. Patients under the age of 44 had higher collected CD34+ cell counts (0.96+/-1.37x10(6) cells/kg) but lower counts of MNC (1.49+/-0.74x10(8) cells/kg).
CONCLUSIONS
The collected MNC and CD34+ cell counts varied between the types of malignancies, and with respect to sex and age. However, only collected MNC counts were significantly different (P<0.05) among the different types of malignancies.
Keyword
MeSH Terms
-
Anesthesia, General
Autografts*
Cell Count
Cell Transplantation
Daegu
Diagnosis
Female
Hematologic Neoplasms*
Humans
Leukemia, Myeloid, Acute
Lymphoma, B-Cell
Lymphoma, T-Cell
Male
Multiple Myeloma
Precursor Cell Lymphoblastic Leukemia-Lymphoma
Retrospective Studies
Stem Cells*
T-Lymphocytes
Transplantation, Autologous*
Transplants
Figure
Reference
-
References
1. Winters JL. Hemapheresis. Henry JB, editor. Clinical diagnosis and management by laboratory methods. 22th ed.Philadelphia: WB Saunders;2006. p. 746–73.
Article2. Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006; 354:1813–26.
Article3. Siena S, Bregni M, Brando B, Belli N, Ravagnani F, Gandola L, et al. Flow cytometry for clinical estimation of circulating hematopoietic progenitors for autologous transplantation in cancer patients. Blood. 1991; 77:400–9.
Article4. Lee J, Lee MH, Park KW, Kang JH, Im DH, Kim K, et al. Influential factors for the collection of peripheral blood stem cells and engraftment in acute myeloid leukemia patients in first complete remission. Int J Hematol. 2005; 81:258–63.
Article5. Beutler E, Lichtman MA, et al. eds. Principle of hematopoietic cell transplantation. 7th ed.William Hematol: McGraw-Hill;2006. p. 301–22.6. Bensinger W, Appelbaum F, Rowley S, Storb R, Sanders J, Lilleby K, et al. Factors that influence collection and engraftment of autologous pe-ripheral-blood stem cells. J Clin Oncol. 1995; 13:2547–55.
Article7. Schwartzberg L, Birch R, Blanco R, Wittlin F, Muscato J, Tauer K, et al. Rapid and sustained hematopoietic reconstitution by peripheral blood stem cell infusion alone following high-dose chemotherapy. Bone Marrow Transplant. 1993; 11:369–74.8. Stewart DA, Guo D, Morris D, Poon MC, Ruether BA, Jones AR, et al. Superior autologous blood stem cell mobilization from dose-intensive cyclophosphamide, etoposide, cisplatin plus G-CSF than from less intensive chemotherapy regimens. Bone Marrow Transplant. 1999; 23:111–7.
Article9. Jowitt SN, Chang J, Morgenstern GR, Howe T, Ryder WD, Testa NG, et al. Factors which affect the CFU-GM content of the peripheral blood haemopoietic progenitor cell harvests in patients with acute myeloid leukaemia. Br J Haematol. 1998; 100:688–94.
Article10. Moskowitz CH, Glassman JR, Wuest D, Maslak P, Reich L, Gucciardo A, et al. Factors affecting mobilization of peripheral blood progenitor cells in patients with lymphoma. Clin Cancer Res. 1998; 4:311–6.11. Linker CA, Ries CA, Damon LE, Sayre P, Navarro W, Rugo HS, et al. Autologous stem cell transplantation for acute myeloid leukemia in first remission. Biol Blood Marrow Transplant. 2000; 6:50–7.
Article12. Watts MJ, Sullivan AM, Jamieson E, Pearce R, Fielding A, Devereux S, et al. Progenitor-cell mobilization after low-dose cyclophosphamide and granulocyte colonystimulating factor: an analysis of progenitor-cell quantity and quality and factors predicting for these parameters in 101 pretreated patients with malignant lymphoma. J Clin Oncol. 1997; 15:535–46.
Article13. McQuaker IG, Haynes AP, Stainer C, Anderson S, Russell NH. Stem cell mobilization in resistant or relapsed lymphoma: superior yield of progenitor cells following a salvage regimen comprising ifosphamide, etoposide and epirubicin compared to intermediate-dose cyclophosphamide. Br J Haematol. 1997; 98:228–33.
Article14. Lee RG, Foerster J, et al. eds. Wintrobe's Clinical Hematology. 10th ed.Hematol Malignancies: Mass Publishing Co;1999. p. 1993–2725.15. Craig W, Kay R, Cutler RL, Lansdorp PM. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993; 177:1331–42.
Article16. Uchida N, Combs J, Chen S, Zanjani E, Hoffman R, Tsukamoto A. Primitive human hematopoietic cells displaying differential efflux of the rhodamine 123 dye have distinct biological activities. Blood. 1996; 88:1297–305.
Article17. Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988; 6:1562–8.
Article18. Shpall EJ, Champlin R, Glaspy JA. Effect of CD34+ peripheral blood progenitor cell dose on hematopoietic recovery. Biol Blood Marrow Transplant. 1998; 4:84–92.
Article19. Chae YS, Jeon SB, Sung WJ, Lim JW, Kim DH, Kim JG, et al. Clinical outcomes according to transplanted CD34+ cell dose in allogeneic peripheral blood stem cell transplantation. Korean J Hematol. 2003; 38:24–31.20. Woo HY, Kim HR, Seong KW, Lee HK, Oh WI, Kim DW. Correlation between progenitor cell dose and the rate of engraftment in autologous peripheral blood and allogeneic bone marrow transplantation. Korean J Blood Transfus. 2000; 11:35–47.21. Reddy RL. Mobilization and collection of peripheral blood progenitor cells for transplantation. Transfus Apher Sci. 2005; 32:63–72.
Article22. Filshie RJ. Cytokines in haemopoietic progenitor mobilisation for peripheral blood stem cell transplantation. Curr Pharm Des. 2002; 8:379–394.
Article23. Nowrousian MR, Waschke S, Bojko P, Welt A, Schuett P, Ebeling P, et al. Impact of chemotherapy regimen and hematopoietic growth factor on mobilization and collection of peripheral blood stem cells in cancer patients. Ann Oncol. 2003; 14(S1):i29–36.
Article24. Zhang C, Chen X, Zhang X, Gao L, Kong P, Wang Q, et al. Mobilization of peripheral blood stem cells for autologous transplantation patients with hematological malignancies: Influence of disease, mobilization method, age and sex. Transfus Apher Sci. 2008; 39:21–8.
Article25. Carral A, de la Rubia J, Martín G, Martínez J, Sanz G, Jarque I, et al. Factors influencing hematopoietic recovery after autologous blood stem cell transplantation in patients with acute myeloblastic leukemia and with non-myeloid malignancies. Bone Marrow Transplant. 2002; 29:825–32.
Article26. Ergene U, Cağirgan S, Pehlivan M, Yilmaz M, Tombuloğlu M. Factors influencing engraftment in autologous peripheral hematopoetic stem cell transplantation (PBSCT). Transfus Apher Sci. 2007; 36:23–9.
Article27. Jeung KJ, Kang MS, Kwon KD, Kim KH, Lee JC, Lee SC, et al. Influential factors for engraftment in autologous peripheral hematopoietic stem cell transplantation (APBSCT). Korean J Hematol. 2007; 42:301–8.
Article28. Neben S, Hellman S, Montgomery M, Ferrara J, Mauch P. Hematopoietic stem cell deficit of transplanted bone marrow previously exposed to cytotoxic agents. Exp Hematol. 1993; 21:156–62.29. Morris CL, Siegel E, Barlogie B, Cottler-Fox M, Lin P, Fassas A, et al. Mobilization of CD34+ cells in elderly patients (>/= 70 years) with multiple myeloma: influence of age, prior therapy, platelet count and mobilization regimen. Br J Haematol. 2003; 120:413–23.30. de la Rubia J, Arbona C, de Arriba F, del Cañizo C, Brunet S, Zamora C, et al. Analysis of factors associated with low peripheral blood progenitor cell collection in normal donors. Transfusion. 2002; 42:4–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Scleroderma following Autologous Peripheral Stem Cell Transplantation
- Effect of Fixed Dose of G-CSF on Mobilization and Engraftment of Peripheral Blood Stem Cells in High Dose Chemotherapy
- Factors Predicting Peripheral Blood Stem Cell Collection: Analysis of Korean Patients at a Single Center
- The Correlation between G-CSF Dosage and the Number of Collected CD34+ Cells in Pediatric Patients Undergoing Peripheral Blood Stem Cell Mobilization
- Bendamustine, etoposide, and dexamethasone to mobilize peripheral blood hematopoietic stem cells for autologous transplantation in non-Hodgkin lymphoma