Lab Med Online.
2013 Oct;3(4):269-276.
Beyond Diagnostic Accuracy: The Clinical Utility of Diagnostic Tests
- Affiliations
-
- 1Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Academic Medical Center, University of Amsterdam, Amsterdam, the Netherlands.
- 2Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands.
- 3Section of Forensic Chemistry, Department of Forensic Medicine, Faculty of Health Sciences, University of Copenhagen, Copenhagen, Denmark.
Abstract
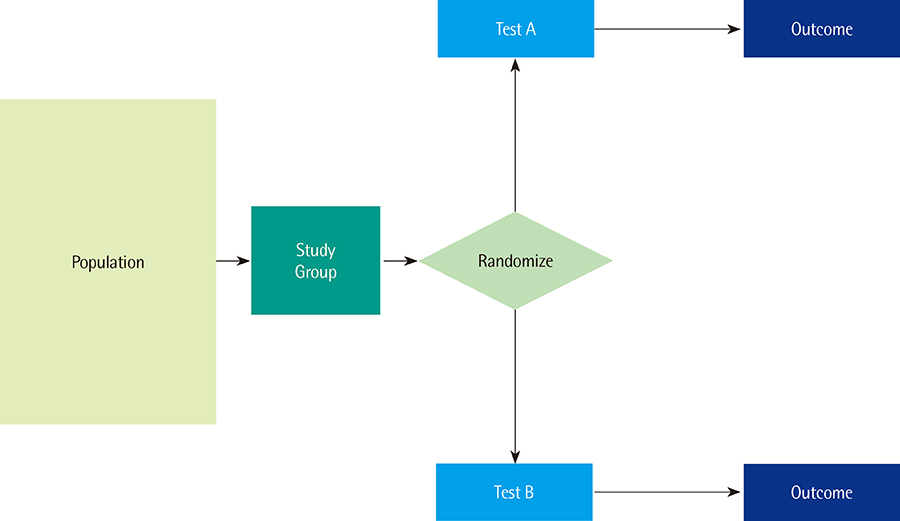
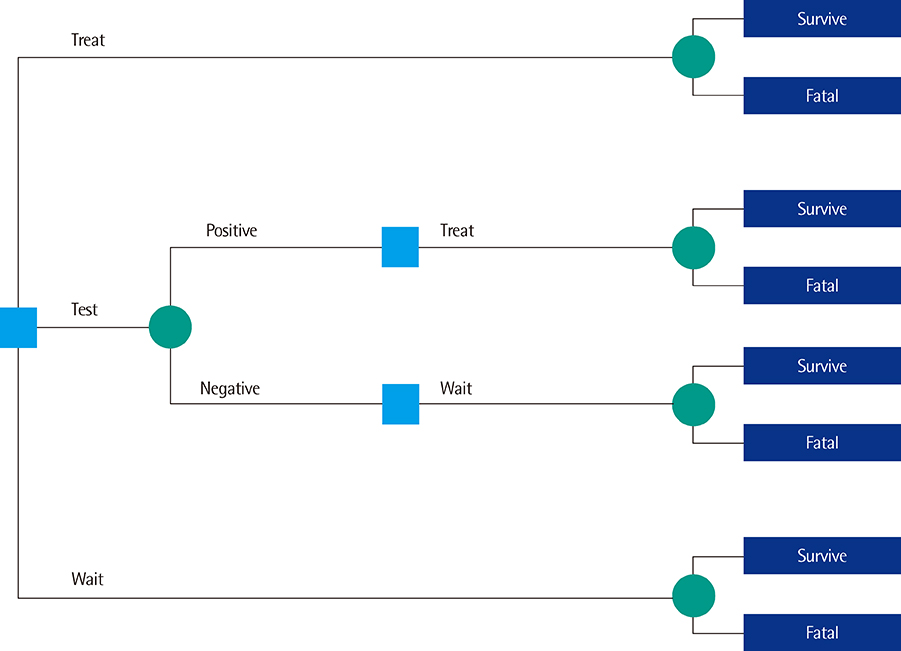
- Like any other medical technology or intervention, diagnostic tests should be thoroughly evaluated before their introduction into daily practice. Increasingly, decision makers, physicians, and other users of diagnostic tests request more than simple measures of a test's analytical or technical performance and diagnostic accuracy; they would also like to see testing lead to health benefits. In this last article of our series, we introduce the notion of clinical utility, which expresses-preferably in a quantitative form-to what extent diagnostic testing improves health outcomes relative to the current best alternative, which could be some other form of testing or no testing at all. In most cases, diagnostic tests improve patient outcomes by providing information that can be used to identify patients who will benefit from helpful downstream management actions, such as effective treatment in individuals with positive test results and no treatment for those with negative results. We describe how comparative randomized clinical trials can be used to estimate clinical utility. We contrast the definition of clinical utility with that of the personal utility of tests and markers. We show how diagnostic accuracy can be linked to clinical utility through an appropriate definition of the target condition in diagnostic-accuracy studies.
Figure
Reference
-
1. Linnet K, Bossuyt PM, Moons KG, Reitsma JB. Quantifying the accuracy of a diagnostic test or marker. Clin Chem. 2012; 58:1292–1301.
Article2. Reitsma JB, Moons KG, Bossuyt PM, Linnet K. Systematic reviews of studies quantifying the accuracy of diagnostic tests and markers. Clin Chem. 2012; 58:1534–1545.
Article3. Moons KG, de Groot JA, Linnet K, Reitsma JB, Bossuyt PM. Quantifying the added value of a diagnostic test or marker. Clin Chem. 2012; 58:1408–1417.
Article4. Neumann PJ, Tunis SR. Medicare and medical technology--the growing demand for relevant outcomes. N Engl J Med. 2010; 362:377–379.
Article5. Lijmer JG, Leeflang M, Bossuyt PM. Proposals for a phased evaluation of medical tests. Med Decis Making. 2009; 29:E13–E21.
Article6. Gazelle GS, Kessler L, Lee DW, McGinn T, Menzin J, Neumann PJ, et al. A framework for assessing the value of diagnostic imaging in the era of comparative effectiveness research. Radiology. 2011; 261:692–698.
Article7. Bossuyt PM, McCaffery K. Additional patient outcomes and pathways in evaluations of testing. Med Decis Making. 2009; 29:E30–E38.
Article8. Marteau TM, Weinman J. Self-regulation and the behavioural response to DNA risk information: a theoretical analysis and framework for future research. Soc Sci Med. 2006; 62:1360–1368.
Article9. Heitman SJ, Hilsden RJ, Au F, Dowden S, Manns BJ. Colorectal cancer screening for average-risk North Americans: an economic evaluation. PLoS Med. 2010; 7:e1000370.
Article10. Lord SJ, Irwig L, Simes RJ. When is measuring sensitivity and specificity sufficient to evaluate a diagnostic test, and when do we need randomized trials? Ann Intern Med. 2006; 144:850–855.
Article11. Bossuyt PM, Lijmer JG, Mol BW. Randomised comparisons of medical tests: sometimes invalid, not always efficient. Lancet. 2000; 356:1844–1847.
Article12. Biesheuvel CJ, Grobbee DE, Moons KG. Distraction from randomization in diagnostic research. Ann Epidemiol. 2006; 16:540–544.
Article13. Hunink MG, Krestin GP. Study design for concurrent development, assessment, and implementation of new diagnostic imaging technology. Radiology. 2002; 222:604–614.
Article14. Kelloff GJ, Sigman CC. Cancer biomarkers: selecting the right drug for the right patient. Nat Rev Drug Discov. 2012; 11:201–214.
Article15. Wagner JA, Williams SA, Webster CJ. Biomarkers and surrogate end points for fit-for-purpose development and regulatory evaluation of new drugs. Clin Pharmacol Ther. 2007; 81:104–107.
Article16. Foster MW, Mulvihill JJ, Sharp RR. Evaluating the utility of personal genomic information. Genet Med. 2009; 11:570–574.
Article17. Grosse SD, Khoury MJ. What is the clinical utility of genetic testing? Genet Med. 2006; 8:448–450.
Article18. Pletcher MJ, Pignone M. Evaluating the clinical utility of a biomarker: a review of methods for estimating health impact. Circulation. 2011; 123:1116–1124.19. Institute of Medicine (US) Roundtable on Translating Genomic-Based Research for Health. Wizemann T, Berger AC, editors. The value of genetic and genomic technologies: workshop summary. Washington, DC: National Academies of Press/Institute of Medicine;2010.20. Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004; 350:647–654.
Article21. Breidthardt T, Laule K, Strohmeyer AH, Schindler C, Meier S, Fischer M, et al. Medical and economic long-term effects of B-type natriuretic peptide testing in patients with acute dyspnea. Clin Chem. 2007; 53:1415–1422.
Article22. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009; 119:2408–2416.
Article23. Moons KG. Criteria for scientific evaluation of novel markers: a perspective. Clin Chem. 2010; 56:537–541.
Article24. Clarke LD, Plevritis SK, Boer R, Cronin KA, Feuer EJ. A comparative review of CISNET breast models used to analyze U.S. breast cancer incidence and mortality trends. J Natl Cancer Inst Monogr. 2006; 36:96–105.25. Lord SJ, Staub LP, Bossuyt PM, Irwig LM. Target practice: choosing target conditions for test accuracy studies that are relevant to clinical practice. BMJ. 2011; 343:d4684.
Article26. Price CP, Christenson RH. Evaluating new diagnostic technologies: perspectives in the UK and US. Clin Chem. 2008; 54:1421–1423.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Meta-Analysis of Diagnostic Test Accuracy
- Overview of the Process of Conducting Meta-analyses of the Diagnostic Test Accuracy
- Methods for Evaluating the Accuracy of Diagnostic Tests
- An overview of systematic reviews of diagnostic tests accuracy
- Percutaneous Needle Biopsy of Bony Lesions: Diagnostic Accuracy and Clinical Utility