Lab Anim Res.
2015 Dec;31(4):180-187. 10.5625/lar.2015.31.4.180.
Supraphysiologic glucocorticoid administration increased biomechanical bone strength of rats' vertebral body
- Affiliations
-
- 1Department of Biology and Anatomical Sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 2Department of Anatomy, School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran.
- 3Celluar and Molecular Biology Research Centre, Shahid Beheshti University of Medical Sciences, Tehran, Iran. mohbayat@sbmu.ac.ir
- 4School of Medicine, Arak University of Medical Sciences, Arak, Iran.
- KMID: 2312144
- DOI: http://doi.org/10.5625/lar.2015.31.4.180
Abstract
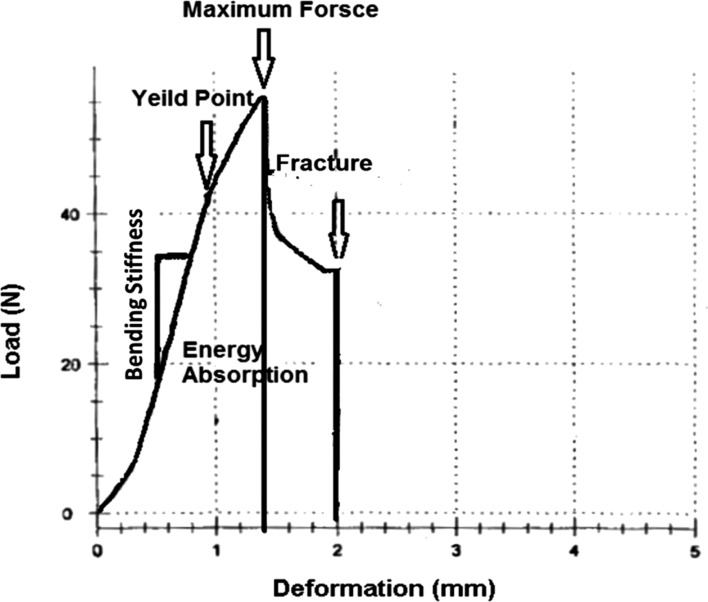
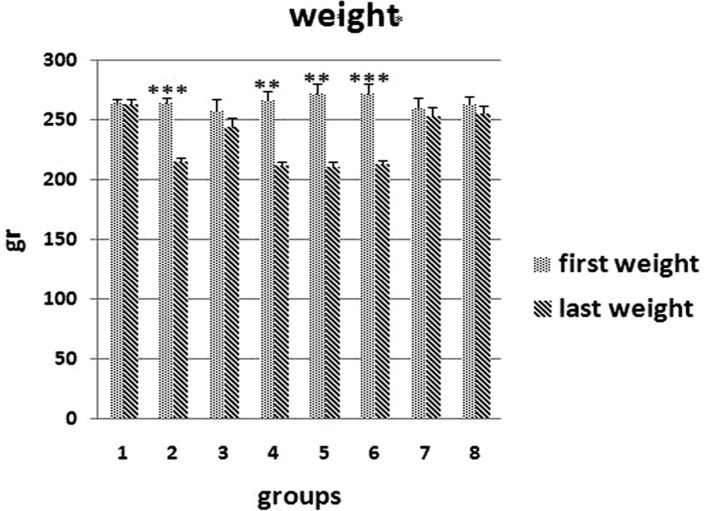
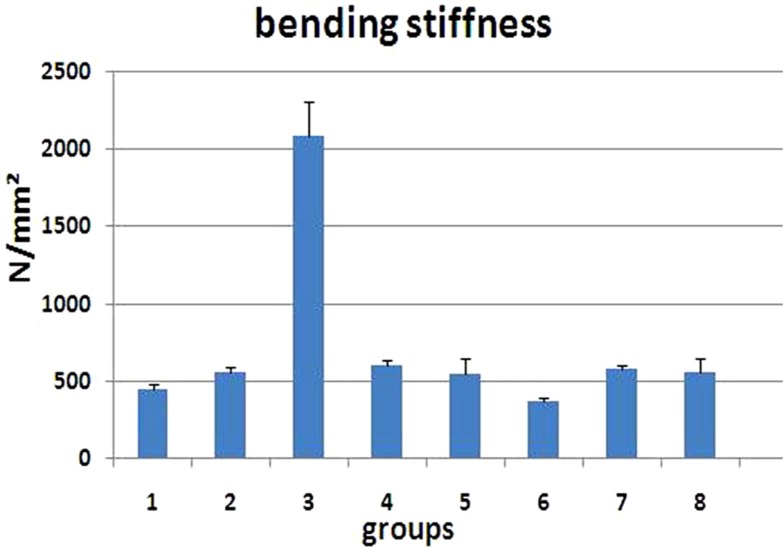
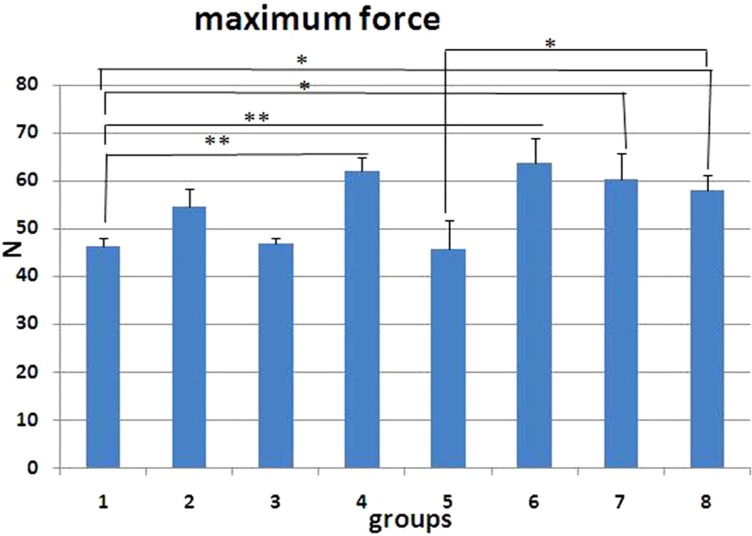
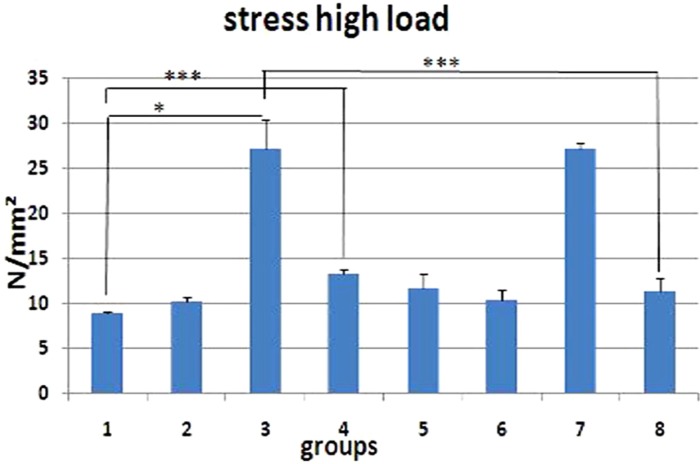
- The aim of this study is to assess the effects of different glucocorticoid administration protocols on biomechanical properties of the first lumbar vertebral body in rats. We divided 40 male rats into the following groups: control, dexamethasone (7 mg/week), dexamethasone (0.7 mg/week), methylprednisolone (7 mg/kg/week), methylprednisolone (5 mg/kg twice weekly), dexamethasone (7 mg/kg three times per week), dexamethasone (0.7 mg/kg three times per week, and low-level laser treated rats. Lumbar vertebrae in rats were exposed to the pulsed laser. We conducted a biomechanical test to examine the mechanical properties of vertebral body in rats' lumbar bone. Supraphysiologic glucocorticoid administration protocols did not impair the biomechanical properties of rats' vertebral bodies compared to control and laser-treated rats. Supraphysiologic glucocorticoid administration caused an anabolic effect on the vertebral bodies.
Keyword
MeSH Terms
Figure
Reference
-
1. Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B. Mortality after osteoporotic fractures. Osteoporos Int. 2004; 15(1):38–42. PMID: 14593451.
Article2. Kim HJ. New understanding of glucocorticoid action in bone cells. BMB Rep. 2010; 43(8):524–529. PMID: 20797313.
Article3. Popp AW, Isenegger J, Buergi EM, Buergi U, Lippuner K. Glucocorticosteroid-induced spinal osteoporosis: scientific update on pathophysiology and treatment. Eur Spine J. 2006; 15(7):1035–1049. PMID: 16474946.
Article4. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013; 65(2):294–298. PMID: 22807233.
Article5. Egermann M, Goldhahn J, Schneider E. Animal models for fracture treatment in osteoporosis. Osteoporos Int. 2005; 16(2 S):S129–S138. PMID: 15750681.
Article6. Peng Z, Tuukkanen J, Zhang H, Jämsä T, Väänänen HK. The mechanical strength of bone in different rat models of experimental osteoporosis. Bone. 1994; 15(5):523–532. PMID: 7980963.
Article7. Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993; 14(4):595–608. PMID: 8274302.
Article8. Binz K, Schmid C, Bouillon R, Froesch ER, Jürgensen K, Hunziker EB. Interactions of insulin-like growth factor I with dexamethasone on trabecular bone density and mineral metabolism in rats. Eur J Endocrinol. 1994; 130(4):387–393. PMID: 8162170.9. Li M, Shen Y, Halloran BP, Baumann BD, Miller K, Wronski TJ. Skeletal response to corticosteroid deficiency and excess in growing male rats. Bone. 1996; 19(2):81–88. PMID: 8853849.
Article10. Shen V, Birchman R, Liang XG, Wu DD, Lindsay R, Dempster DW. Prednisolone alone, or in combination with estrogen or dietary calcium deficiency or immobilization, inhibits bone formation but does not induce bone loss in mature rats. Bone. 1997; 21(4):345–351. PMID: 9315338.
Article11. Wimalawansa SJ, Chapa MT, Yallampalli C, Zhang R, Simmons DJ. Prevention of corticosteroid-induced bone loss with nitric oxide donor nitroglycerin in male rats. Bone. 1997; 21(3):275–280. PMID: 9276093.
Article12. Ortoft G, Oxlund H, Andreassen TT. Administration of a glucocorticoid with depot effect counteracts the stimulating effect of growth hormone on cancellous and cortical bone of the vertebral body in rats. Calcif Tissue Int. 1998; 63(1):14–21. PMID: 9632841.13. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998; 102(2):274–282. PMID: 9664068.
Article14. Weinstein RS, Chen JR, Powers CC, Stewart SA, Landes RD, Bellido T, Jilka RL, Parfitt AM, Manolagas SC. Promotion of osteoclast survival and antagonism of bisphosphonate-induced osteoclast apoptosis by glucocorticoids. J Clin Invest. 2002; 109(8):1041–1048. PMID: 11956241.
Article15. Hulley PA, Conradie MM, Langeveldt CR, Hough FS. Glucocorticoid-induced osteoporosis in the rat is prevented by the tyrosine phosphatase inhibitor, sodium orthovanadate. Bone. 2002; 31(1):220–229. PMID: 12110438.
Article16. McLaughlin F, Mackintosh J, Hayes BP, McLaren A, Uings IJ, Salmon P, Humphreys J, Meldrum E, Farrow SN. Glucocorticoid-induced osteopenia in the mouse as assessed by histomorphometry, microcomputed tomography, and biochemical markers. Bone. 2002; 30(6):924–930. PMID: 12052464.
Article17. Conradie MM, de Wet H, Kotze DD, Burrin JM, Hough FS, Hulley PA. Vanadate prevents glucocorticoid-induced apoptosis of osteoblasts in vitro and osteocytes in vivo. J Endocrinol. 2007; 195(2):229–240. PMID: 17951534.18. Lucinda LM, Vieira BJ, Oliveira TT, Sá RC, Peters VM, Reis JE, Guerra MO. Evidences of osteoporosis improvement in Wistar rats treated with Ginkgo biloba extract: a histomorphometric study of mandible and femur. Fitoterapia. 2010; 81(8):982–987. PMID: 20600689.
Article19. Sun P, Cai DH, Li QN, Chen H, Deng WM, He L, Yang L. Effects of alendronate and strontium ranelate on cancellous and cortical bone mass in glucocorticoid-treated adult rats. Calcif Tissue Int. 2010; 86(6):495–501. PMID: 20390406.
Article20. Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, Kirilov M, Mandic V, Takacz A, Schmidt-Ullrich R, Ostermay S, Schinke T, Spanbroek R, Zaiss MM, Angel PE, Lerner UH, David JP, Reichardt HM, Amling M, Schütz G, Tuckermann JP. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 2010; 11(6):517–531. PMID: 20519123.
Article21. Weinstein RS, O'Brien CA, Almeida M, Zhao H, Roberson PK, Jilka V, Manolagas SC. Osteoprotegerin prevents glucocorticoidinduced osteocyte apoptosis in mice. Endocrinology. 2011; 152(9):3323–3331. PMID: 21771887.
Article22. Cui L, Li T, Liu Y, Zhou L, Li P, Xu B, Huang L, Chen Y, Liu Y, Tian X, Jee WS, Wu T. Salvianolic acid B prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis. PLoS One. 2012; 7(4):e34647. PMID: 22493705.
Article23. Pennisi P, D'Alcamo MA, Leonetti C, Clementi A, Cutuli VM, Riccobene S, Parisi N, Fiore CE. Supplementation of L-arginine prevents glucocorticoid-induced reduction of bone growth and bone turnover abnormalities in a growing rat model. J Bone Miner Metab. 2005; 23(2):134–139. PMID: 15750691.
Article24. Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, Kostenuik PJ, Erben RG, Hofbauer LC. Inhibition of receptor activator of NF-kappa B ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009; 175(2):473–478. PMID: 19590040.25. Yoon HY, Won YY, Chung YS. Poncirin prevents bone loss in glucocorticoid-induced osteoporosis in vivo and in vitro. J Bone Miner Metab. 2012; 3(5):509–516. PMID: 22407507.26. Reddy GK. Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg. 2004; 22(2):141–150. PMID: 15165389.
Article27. Lewiecki EM, Laster AJ. Clinical applications of vertebral fracture assessment by dual-energy x-ray absorptiometry. J Clin Endocrinol Metab. 2006; 91(11):4215–4222. PMID: 16940447.
Article28. Green D, Wallace H. Late effects of childhood cancer. 2003. CRC Press.29. Freidouni M, Nejati H, Salimi M, Bayat M, Amini A, Noruzian M, Asgharie MA, Rezaian M. Evaluating glucocorticoid administration on biomechanical properties of rats' tibial diaphysis. Iran Red Crescent Med J. 2015; 17(3):e19389. PMID: 26019900.
Article30. Fridoni M, Masteri Farahani R, Nejati H, Salimi M, Gharavi SM, Bayat M, Amini A, Torkman G, Bayat S. Evaluation of the effects of LLLT on biomechanical properties of tibial diaphysis in two rat models of experimental osteoporosis by a three point bending test. Lasers Med Sci. 2015; 30(3):1117–1125. PMID: 25616711.
Article31. Dadpay M, Sharifian Z, Bayat M, Bayat M, Dabbagh A. Effects of pulsed infra-red low level-laser irradiation on open skin wound healing of healthy and streptozotocin-induced diabetic rats by biomechanical evaluation. J Photochem Photobiol B. 2012; 111:1–8. PMID: 22494918.
Article32. Bayat M, Abdi S, Javadieh F, Mohsenifar Z, Rashid MR. The effects of low-level laser therapy on bone in diabetic and nondiabetic rats. Photomed Laser Surg. 2009; 27(5):703–708. PMID: 19698018.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Growth Hormone on Steroid-induced Musculoskeletal Changes of Rats
- Biomechanical Evaluation of the Pullout Strength of the Dynamic Osteosynthesis Construct(DOC) Anterior Cervical Plating System: A Comparison between the Screw Angulations to Consider the Concept of Triangulation
- An Experimental Study about the Effects of Parathyroid Hormone on Strength of Vertebra and Bone Density of Proximal Tibia in Ovariectomized Rats
- Analysis of bone in adenine-induced chronic kidney disease model rats
- Combined treatment with minodronate and vitamin C increases bone mineral density and strength in vitamin C-deficient rats