Allergy Asthma Immunol Res.
2016 Sep;8(5):412-420. 10.4168/aair.2016.8.5.412.
Short-, Intermediate-, and Long-Term Changes in Basophil Reactivity Induced by Venom Immunotherapy
- Affiliations
-
- 1Ciudad de Coria Hospital, Allergy Department, Cáceres, Spain. ana.rodriguez@ses.juntaextremadura.net
- 2San Pedro de Alcántara Hospital, Immunology Department, Cáceres Spain.
- 3Department of Mathematics, University of Extremadura, Cáceres, Spain.
- KMID: 2295150
- DOI: http://doi.org/10.4168/aair.2016.8.5.412
Abstract
- PURPOSE
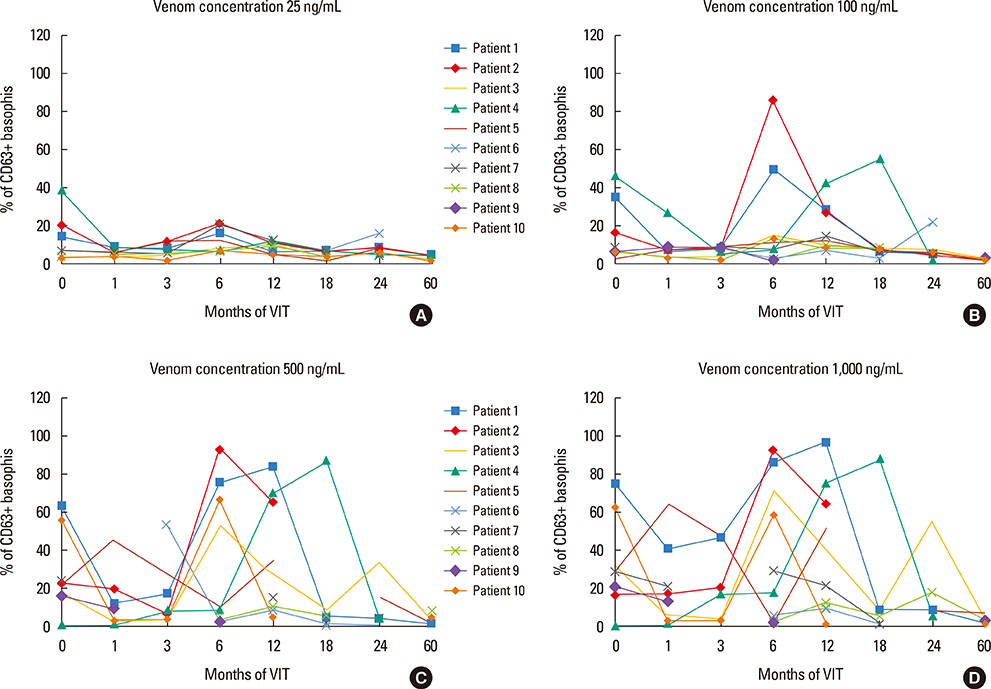
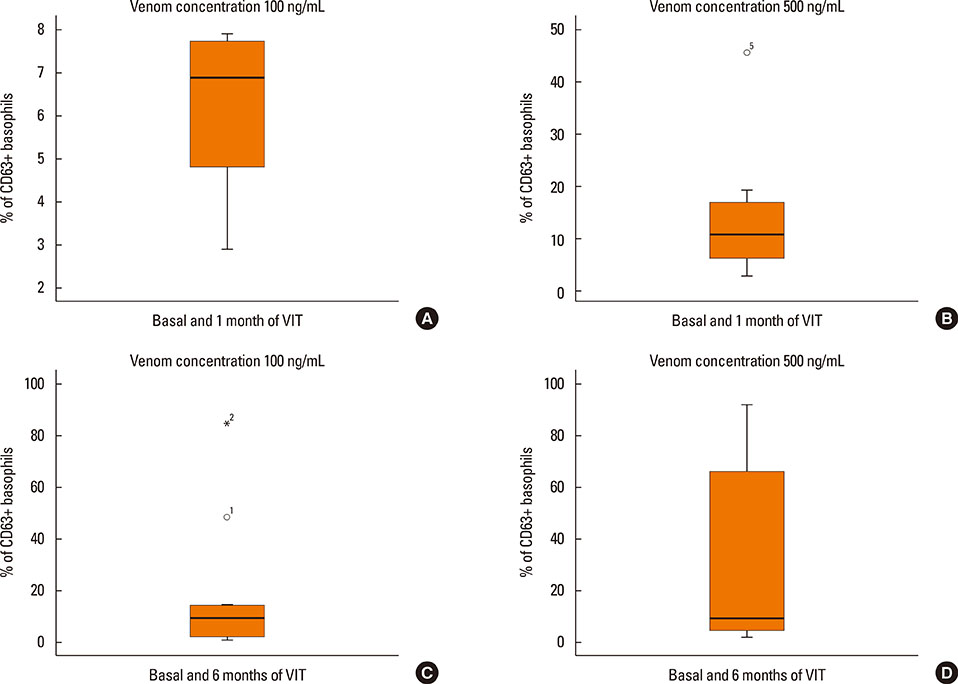
The basophil activation test (BAT) has been used to monitor venom immunotherapy (VIT) due to its high specificity. A previous study has reported a good correlation between a significant decrease in basophil activation during 5 years of VIT and clinical protection assessed by sting challenge. The following prospective study was performed to examine changes in basophil reactivity over a complete VIT period of 5 years.
METHODS
BAT in a dose-response curve was studied prospectively in 10 hymenoptera venom-allergic patients over 5 years of VIT. BAT was performed at the time of diagnosis, 1 month after finishing the VIT build-up phase, and 3, 6, 12, 24, and 60 months after beginning treatment. The repeated measures ANOVA was applied to evaluate basophil activation changes throughout VIT. A cross-sectional study was also performed in 6 patients who received treatment for more than 3 years, and in another 12 patients who followed immunotherapy for at least 5 years.
RESULTS
An early activation decrease was observed during the first 3 months of treatment, compared to pre-treatment values. This activation decrease was not maintained 6 to 18 months after treatment, but was observed again after 2 years of treatment, and maintained until the completion of the 5-year immunotherapy period. In cross-sectional analysis, the 6 patients who received treatment for 3 years, and 9 of the 12 patients who received treatment for 5 years, had negative BAT results. Three patients in this last group had positive BAT results and 2 patients had systemic reactions after field stings.
CONCLUSIONS
BAT appears to be an optimal non-invasive test for close monitoring of VIT.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Changes in basophil activation during immunotherapy with house dust mite and mugwort in patients with allergic rhinitis
Sae-Hoon Kim, Soon-Hee Kim, Soo-Jie Chung, Jung-Hyun Kim, Suh-Young Lee, Byung-Keun Kim, Kyung-Whan Lim, Yoon-Seok Chang
Asia Pac Allergy. 2018;8(1):. doi: 10.5415/apallergy.2018.8.e6.
Reference
-
1. Müller U, Helbling A, Berchtold E. Immunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safety. J Allergy Clin Immunol. 1992; 89:529–535.2. Golden DB. Insect sting allergy and venom immunotherapy: a model and a mystery. J Allergy Clin Immunol. 2005; 115:439–447.3. Ruëff F, Wenderoth A, Przybilla B. Patients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom doses. J Allergy Clin Immunol. 2001; 108:1027–1032.4. Keating MU, Kagey-Sobotka A, Hamilton RG, Yunginger JW. Clinical and immunologic follow-up of patients who stop venom immunotherapy. J Allergy Clin Immunol. 1991; 88:339–348.5. van Halteren HK, van der Linden PW, Burgers JA, Bartelink AK. Discontinuation of yellow jacket venom immunotherapy: follow-up of 75 patients by means of deliberate sting challenge. J Allergy Clin Immunol. 1997; 100:767–770.6. Graft DF. Discontinuing venom immunotherapy. J Allergy Clin Immunol. 1997; 99:271.7. Golden DB, Kwiterovich KA, Kagey-Sobotka A, Lichtenstein LM. Discontinuing venom immunotherapy: extended observations. J Allergy Clin Immunol. 1998; 101:298–305.8. Lerch E, Müller UR. Long-term protection after stopping venom immunotherapy: results of re-stings in 200 patients. J Allergy Clin Immunol. 1998; 101:606–612.9. Ruëff F, Przybilla B, Müller U, Mosbech H. The sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical Immunology. Allergy. 1996; 51:216–225.10. Akdis CA, Blesken T, Akdis M, Wüthrich B. BlaserK. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998; 102:98–106.11. Bussmann C, Xia J, Allam JP, Maintz L, Bieber T, Novak N. Early markers for protective mechanisms during rush venom immunotherapy. Allergy. 2010; 65:1558–1565.12. Konno S, Golden DB, Schroeder J, Hamilton RG, Lichtenstein LM, Huang SK. Increased expression of osteopontin is associated with long-term bee venom immunotherapy. J Allergy Clin Immunol. 2005; 115:1063–1067.13. Dubois AE, de Monchy JG. Sting challenge outcome as a selection criterion for venom immunotherapy. J Allergy Clin Immunol. 1997; 100:144–146.14. Bilò MB, Brianzoni MF, Garritani MS, Antonicelli L, Farabollini B, Bonifazi F. The sting challenge test in Hymenoptera venom allergy: pros and cons. Eur Ann Allergy Clin Immunol. 2003; 35:377–381.15. Sainte-Laudy J, Vallon C, Guérin JC. Analysis of membrane expression of the CD63 human basophil activation marker. Applications to allergologic diagnosis. Allerg Immunol (Paris). 1994; 26:211–214.16. Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry B Clin Cytom. 2008; 74:201–210.17. Rodríguez-Trabado A, Cámara-Hijón C, Ramos-Cantariño A, Porcel-Carreño SL, Jiménez-Timón S, Pereira-Navarro G, et al. Basophil activation test for the in vitro diagnosis of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Proc. 2008; 29:241–249.18. Sturm GJ, Böhm E, Trummer M, Weiglhofer I, Heinemann A, Aberer W. The CD63 basophil activation test in Hymenoptera venom allergy: a prospective study. Allergy. 2004; 59:1110–1117.19. Eberlein-König B, Schmidt-Leidescher C, Rakoski J, Behrendt H, Ring J. In vitro basophil activation using CD63 expression in patients with bee and wasp venom allergy. J Investig Allergol Clin Immunol. 2006; 16:5–10.20. Korosec P, Erzen R, Silar M, Bajrovic N, Kopac P, Kosnik M. Basophil responsiveness in patients with insect sting allergies and negative venom-specific immunoglobulin E and skin prick test results. Clin Exp Allergy. 2009; 39:1730–1737.21. Eberlein-König B, Rakoski J, Behrendt H, Ring J. Use of CD63 expression as marker of in vitro basophil activation in identifying the culprit in insect venom allergy. J Investig Allergol Clin Immunol. 2004; 14:10–16.22. Ebo DG, Hagendorens MM, Bridts CH, De Clerck LS, Stevens WJ. Hymenoptera venom allergy: taking the sting out of difficult cases. J Investig Allergol Clin Immunol. 2007; 17:357–360.23. Erdmann SM, Sachs B, Kwiecien R, Moll-Slodowy S, Sauer I, Merk HF. The basophil activation test in wasp venom allergy: sensitivity, specificity and monitoring specific immunotherapy. Allergy. 2004; 59:1102–1109.24. Ebo DG, Hagendorens MM, Schuerwegh AJ, Beirens LM, Bridts CH, De Clerck LS, et al. Flow-assisted quantification of in vitro activated basophils in the diagnosis of wasp venom allergy and follow-up of wasp venom immunotherapy. Cytometry B Clin Cytom. 2007; 72:196–203.25. Mikkelsen S, Bibby BM, Dolberg MK, Dahl R, Hoffmann HJ. Basophil sensitivity through CD63 or CD203c is a functional measure for specific immunotherapy. Clin Mol Allergy. 2010; 8:2.26. Peternelj A, Silar M, Erzen R, Kosnik M, Korosec P. Basophil sensitivity in patients not responding to venom immunotherapy. Int Arch Allergy Immunol. 2008; 146:248–254.27. Čelesnik N, Vesel T, Rijavec M, Šilar M, Eržen R, Košnik M, et al. Short-term venom immunotherapy induces desensitization of FcεRI-mediated basophil response. Allergy. 2012; 67:1594–1600.28. Eržen R, Košnik M, Silar M, Korošec P. Basophil response and the induction of a tolerance in venom immunotherapy: a long-term sting challenge study. Allergy. 2012; 67:822–830.29. Hausmann O, Diaz C, Schneider M, Weber J, Pecaric-Petkovic T, Helbling A. Correlation of sting challenge outcome and change in EC50 in basophil activation test (BAT) in bee venom allergic patients after 2-5 years of venom immunotherapy. Allergy. 2014; 69:Suppl 99. 111.30. Žitnik SE, Vesel T, Avčin T, Šilar M, Košnik M, Korošec P. Monitoring honeybee venom immunotherapy in children with the basophil activation test. Pediatr Allergy Immunol. 2012; 23:166–172.31. Position paper: Allergen standardization and skin tests. The European Academy of Allergology and Clinical Immunology. Allergy. 1993; 48:48–82.32. Rodríguez Trabado A, Fernández Pereira LM, Romero-Chala S, García-Trujillo JA, Cámara Hijón C. Evaluation of latex subclinical sensitization by way of the basophil activation test and specific IgE to latex recombinant allergens. Allergol Int. 2013; 62:385–387.33. Rodríguez Trabado A, Cámara Hijón C, Porcel Carreño SL, Rodríguez Martín E, Fletes Peral C, Pereira Navarro G, et al. Anaphylaxis caused by cloxacillin: diagnosis with seriated analysis by way of basophil activation test. Allergy Asthma Proc. 2006; 27:269–272.34. Gastaminza G, Algorta J, Uriel O, Audicana MT, Fernandez E, Sanz ML, et al. Randomized, double-blind, placebo-controlled clinical trial of sublingual immunotherapy in natural rubber latex allergic patients. Trials. 2011; 12:191.35. Horak F, Zieglmayer P, Zieglmayer R, Lemell P, Devillier P, Montagut A, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol. 2009; 124:471–477.36. Van Overtvelt L, Baron-Bodo V, Horiot S, Moussu H, Ricarte C, Horak F, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy. 2011; 66:1530–1537.37. Garrido-Fernández S, García BE, Sanz ML, Echechipía S, Lizaso MT, Tabar AI. Are basophil activation and sulphidoleukotriene determination useful tests for monitoring patients with peach allergy receiving sublingual immunotherapy with a Pru p 3-enriched peach extract? J Investig Allergol Clin Immunol. 2014; 24:106–113.38. Lee J, Park CO, Lee KH. Specific immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res. 2015; 7:221–229.39. Akkoc T, Akdis M, Akdis CA. Update in the mechanisms of allergen-specific immunotheraphy. Allergy Asthma Immunol Res. 2011; 3:11–20.40. James LK, Durham SR. Update on mechanisms of allergen injection immunotherapy. Clin Exp Allergy. 2008; 38:1074–1088.41. Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009; 10:697–705.42. Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009; 10:713–720.43. Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009; 10:706–712.44. Jutel M, Müller UR, Fricker M, Rihs S, Pichler WJ, Dahinden C. Influence of bee venom immunotherapy on degranulation and leukotriene generation in human blood basophils. Clin Exp Allergy. 1996; 26:1112–1118.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vitro Biphasic Effect of Honey Bee Venom on Basophils from Screened Healthy Blood Donors
- Changes in basophil activation during immunotherapy with house dust mite and mugwort in patients with allergic rhinitis
- Combination of omalizumab and bee venom immunotherapy: does it work?
- Clinical features of bee venom anaphylaxis
- Influence of conventional house-dust-mite immunotherapy on histamine releasability from the basophil