J Korean Ophthalmol Soc.
2016 Jun;57(6):969-976. 10.3341/jkos.2016.57.6.969.
Influence of RNFL Thickness on Visual Acuity and Visual Field in Bilateral Temporal Optic Atrophy
- Affiliations
-
- 1Department of Ophthalmology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea. jinchoi@paik.ac.kr
- KMID: 2290501
- DOI: http://doi.org/10.3341/jkos.2016.57.6.969
Abstract
- PURPOSE
To investigate the influence of retinal nerve fiber layer (RNFL) thickness on visual acuity and visual field in patients with bilateral temporal optic atrophy.
METHODS
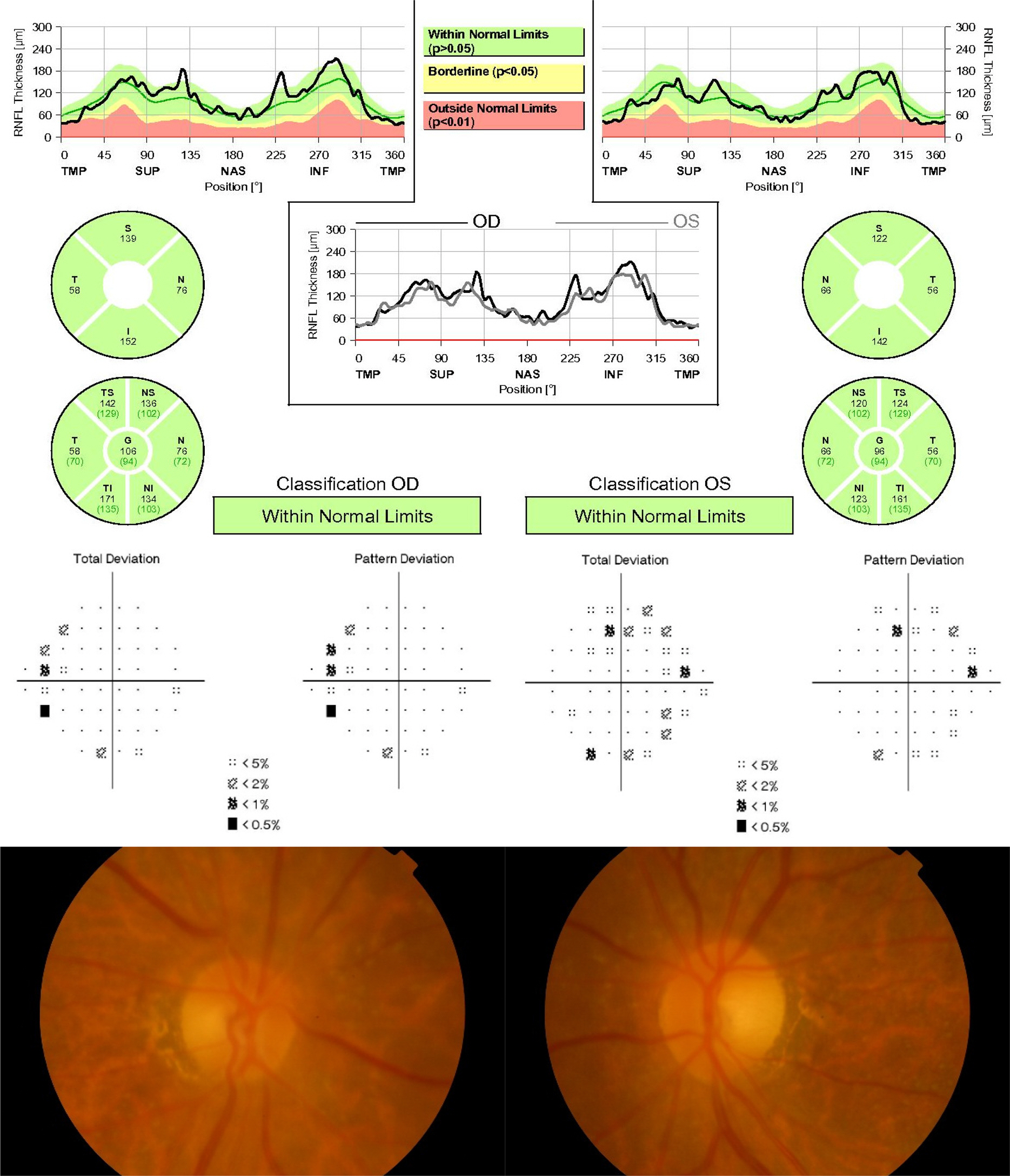
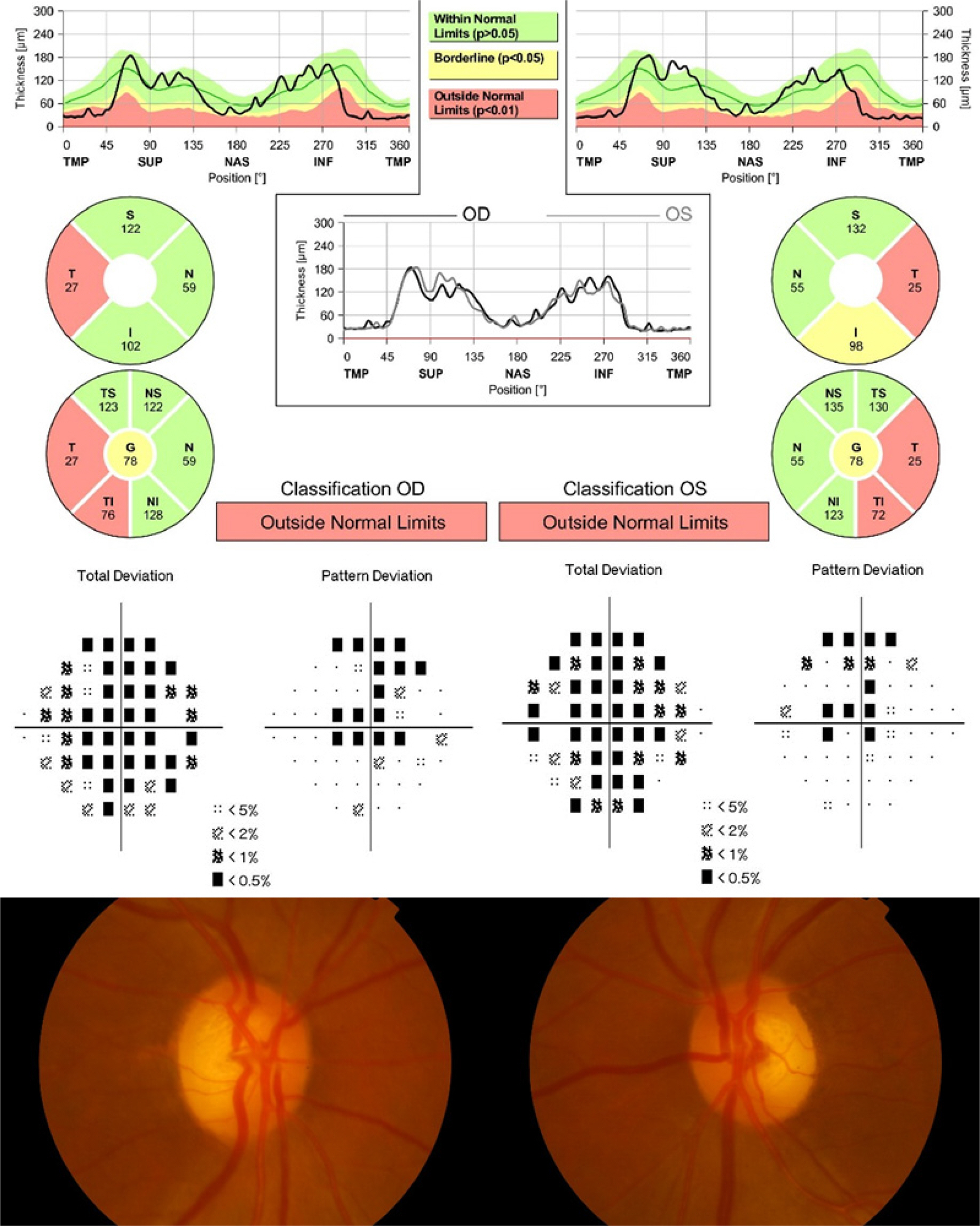
Patients with characteristic features of gradual visual loss and temporal atrophy of both optic nerves were enrolled in this study. Among the patients, RNFL thickness of each area was measured with optical coherence tomography, and its influence on the best corrected visual acuity, mean deviation and pattern standard deviation calculated from the refractive test and Humphrey visual field test was analyzed.
RESULTS
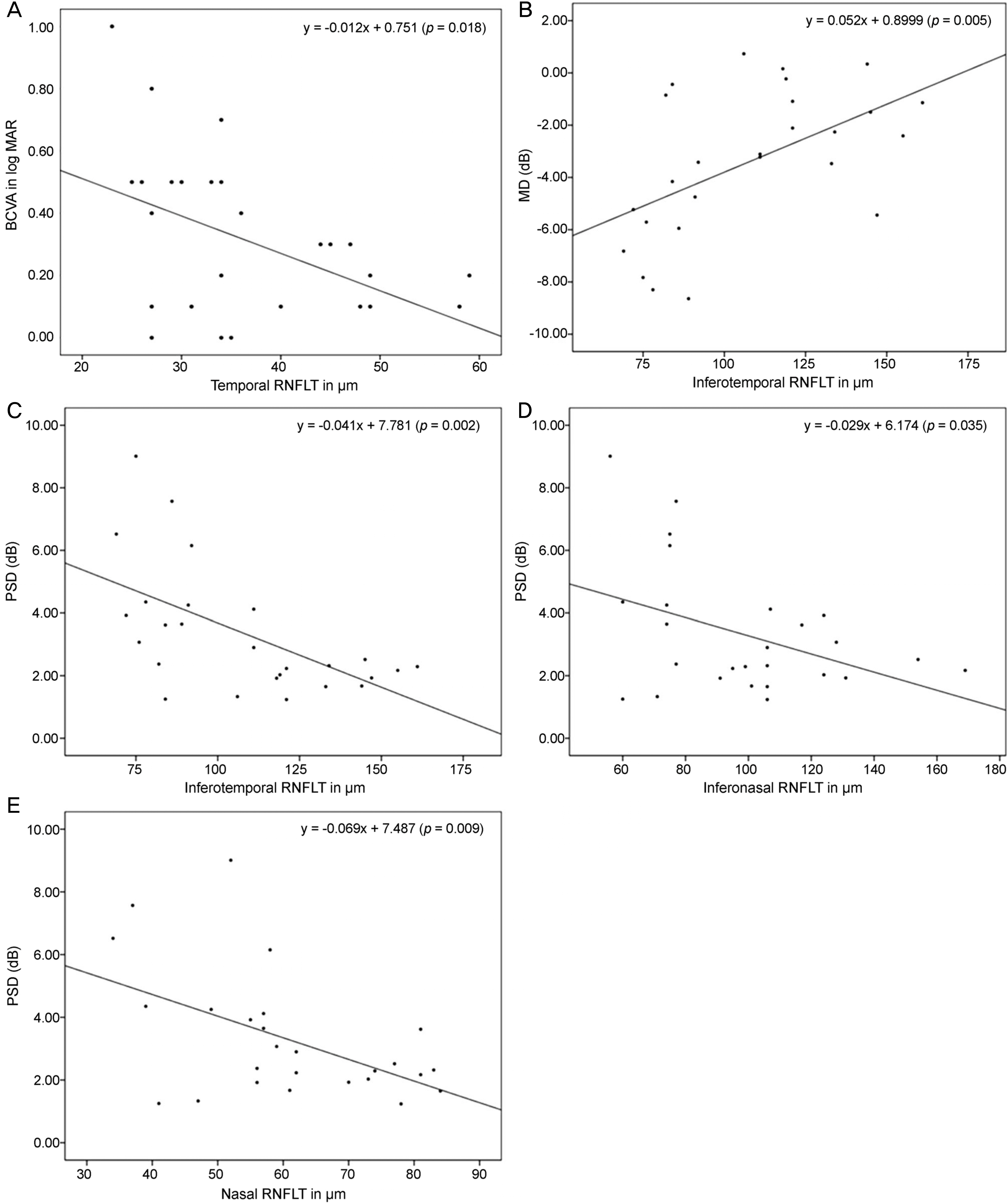
The present study included 13 patients with bilateral temporal optic atrophy (26 eyes) and 13 normal controls (26 eyes). Optical coherence tomography was performed to calculate RNFL thickness in the 52 eyes. Among 26 eyes of patients with bilateral temporal optic atrophy, the Humphrey visual field test was performed to calculate the mean deviation and pattern standard deviation. The mean age in the patient group was 66.0 ± 12.3 years (37-80 years), and 8 (30.8%) patients were male and 18 (69.2%) female. The mean best corrected visual acuity was 30/50 (20/200-20/20). Simple regression analysis showed that a thinner temporal RNFL thickness was correlated with a lower the best corrected visual acuity (p = 0.015). The mean deviation was low when inferotemporal RNFL was thin (p = 0.005). Pattern standard deviation was high when inferotemporal (p = 0.003), inferonasal (p = 0.04) and nasal (p = 0.008) RNFLs were thin.
CONCLUSIONS
Inferotemporal RNFL thickness was significantly correlated with best corrected visual acuity, mean deviation and pattern standard deviation of automated visual field test in patients with bilateral temporal optic neuropathy. Optical coherence tomography can be further used to estimate visual acuity and visual field defects in patients with optic atrophy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Retinal Ganglion Cell Layer Thicknesses and Visual Functions in Patients with Bilateral Temporal Optic Atrophy
Bum Gi Kim, Jae Yong Park, Won Hyuk Oh, Jin Choi
J Korean Ophthalmol Soc. 2020;61(1):92-100. doi: 10.3341/jkos.2020.61.1.92.
Reference
-
References
1. Oluleye TS, Ajaiyeoba AI, Fafowora OF, Olusanya BA. The abdominal of optic atrophy in Nigerians-a general hospital clinic study. Int J Clin Pract. 2005; 59:950–2.2. Vaphiades MS, Brodsky MC. Pediatric optic atrophy. Int Ophthalmol Clin. 2012; 52:17–28. xiii.
Article3. Quigley HA, Anderson DR. The histologic basis of optic disk pallor in experimental optic atrophy. Am J Ophthalmol. 1977; 83:709–17.
Article4. Soltan-Sanjari M, Parvaresh MM, Maleki A, et al. Correlation between retinal nerve fiber layer thickness by optical coherence abdominal and perimetric parameters in optic atrophy. J Ophthalmic Vis Res. 2008; 3:91–4.5. Zhang Y, Huang H, Wei S, et al. Characterization of retinal nerve fiber layer thickness changes associated with Leber's hereditary optic neuropathy by optical coherence tomography. Exp Ther Med. 2014; 7:483–7.
Article6. Kim TW, Hwang JM. Stratus OCT in dominant optic atrophy: abdominals differentiating it from glaucoma. J Glaucoma. 2007; 16:655–8.7. Cardell JD. The aetiology of optic atrophy. Med Press. 1948; 219:139–41.8. Liu T, Bi H, Wang X, et al. Change of retinal nerve fiber layer thickness in patients with nonarteritic inflammatory anterior abdominal optic neuropathy. Neural Regen Res. 2012; 7:2778–83.9. Saxena R, Bandyopadhyay G, Singh D, et al. Evaluation of changes in retinal nerve fiber layer thickness and visual functions in cases of optic neuritis and multiple sclerosis. Indian J Ophthalmol. 2013; 61:562–6.
Article10. Mikelberg FS, Drance SM, Schulzer M, et al. The normal human optic nerve. Axon count and axon diameter distribution. Ophthalmology. 1989; 96:1325–8.11. Park SW, Hwang JM. Optical coherence tomography shows early loss of the inferior temporal quadrant retinal nerve fiber layer in autosomal dominant optic atrophy. Graefes Arch Clin Exp Ophthalmol. 2014; 253:135–41.
Article12. Jansonius NM, Nevalainen J, Selig B, et al. A mathematical abdominal of nerve fiber bundle trajectories and their variability in the human retina. Vision Res. 2009; 49:2157–63.13. Sadun AA, Win PH, Ross-Cisneros FN, et al. Leber's hereditary optic neuropathy differentially affects smaller axons in the optic nerve. Trans Am Ophthalmol Soc. 2000; 98:223–32. discussion 232–5.14. Pan BX, Ross-Cisneros FN, Carelli V, et al. Mathematically abdominal the involvement of axons in Leber's hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2012; 53:7608–17.15. Rebolleda G, Sánchez-Sánchez C, González-López JJ, et al. Papillomacular bundle and inner retinal thicknesses correlate with visual acuity in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2015; 56:682–92.
Article16. Kobayashi W, Kunikata H, Omodaka K, et al. Correlation of abdominal nerve fiber bundle thickness with central visual function in open-angle glaucoma. J Ophthalmol. 2015; 2015:460918.17. Cho KH, Ahn SJ, Jung C, et al. Ischemic injury of the abdominal bundle is a predictive marker of poor vision in eyes with branch retinal artery occlusion. Am J Ophthalmol. 2015; 162:107–20.e2.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retinal Ganglion Cell Layer Thicknesses and Visual Functions in Patients with Bilateral Temporal Optic Atrophy
- Correlation between Visual Acuity and Retinal Nerve Fiber Layer Thickness in Optic Neuropathies
- The Optical Coherence Tomography Findings of Optic Tract Syndrome
- Influence of Epiretinal Membranes on the Retinal Nerve Fiber Layer Thickness Measured by Spectral Domain Optical Coherence Tomography in Glaucoma
- Two Cases of Long-Term Changes in the Retinal Nerve Fiber Layer Thickness after Intravitreal Bevacizumab for Diabetic Papillopathy