Transl Clin Pharmacol.
2016 Jun;24(2):96-104. 10.12793/tcp.2016.24.2.96.
Population pharmacokinetics of imatinib mesylate in healthy Korean subjects
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. yimds@catholic.ac.kr
- 2PIPET (Pharmacometrics Institute for Practical Education and Training), College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2290476
- DOI: http://doi.org/10.12793/tcp.2016.24.2.96
Abstract
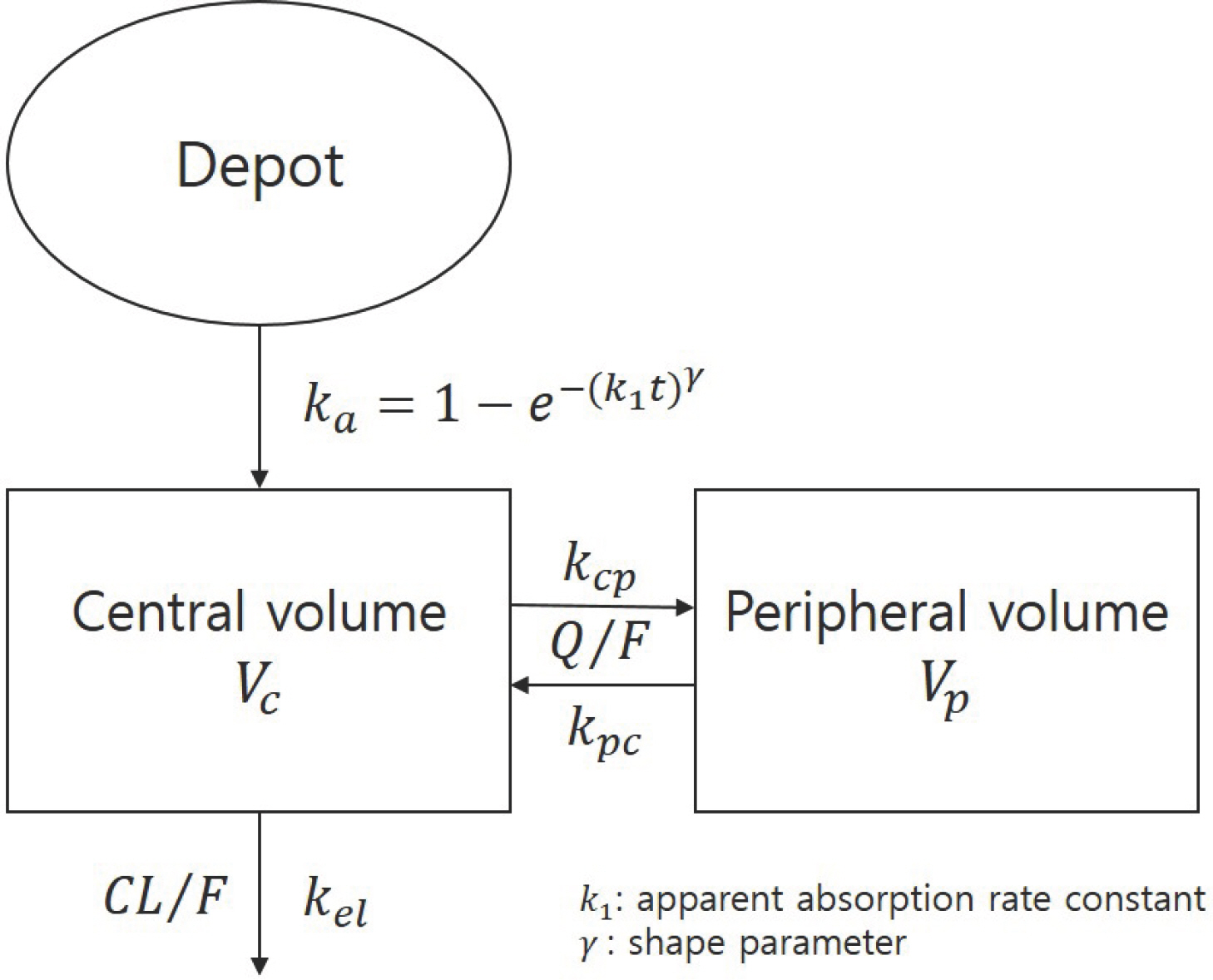
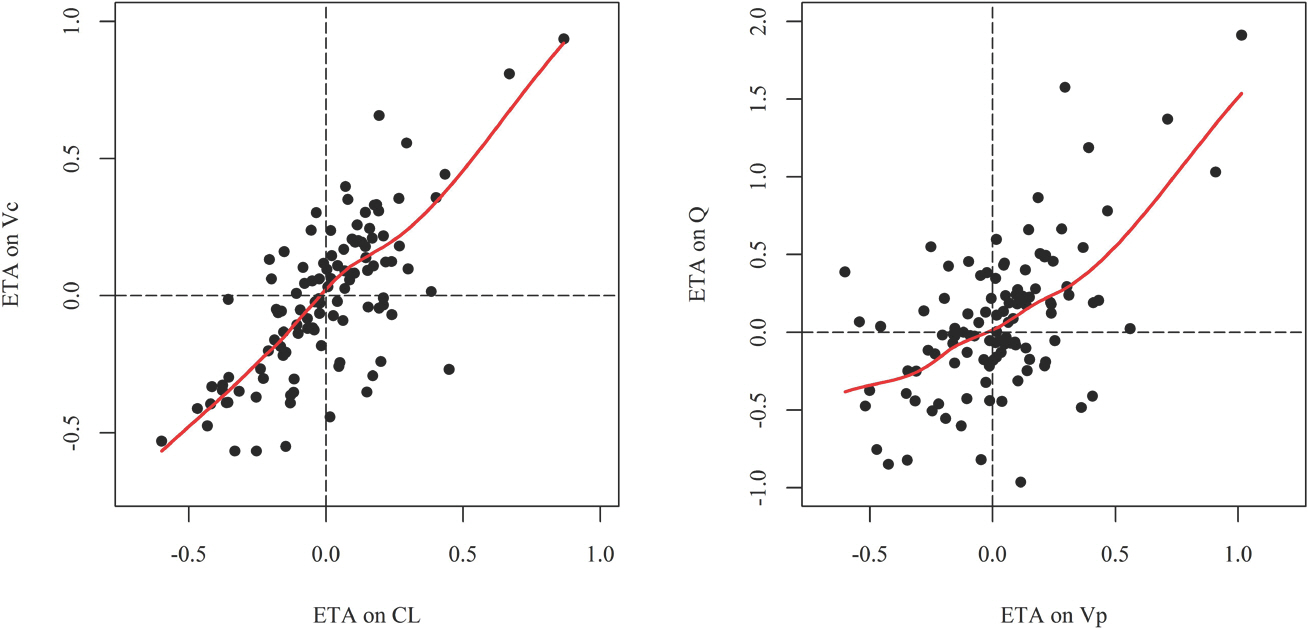
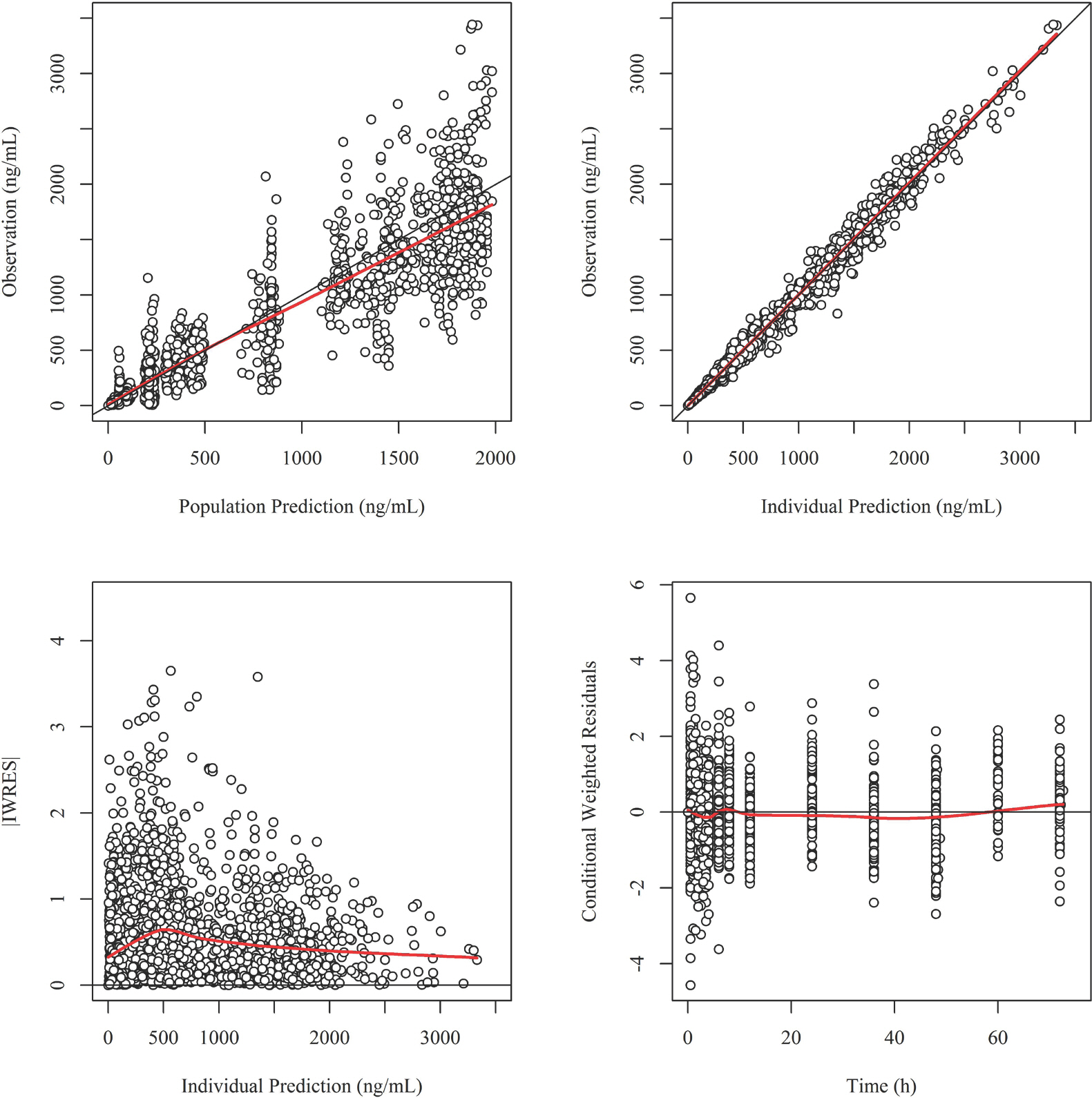
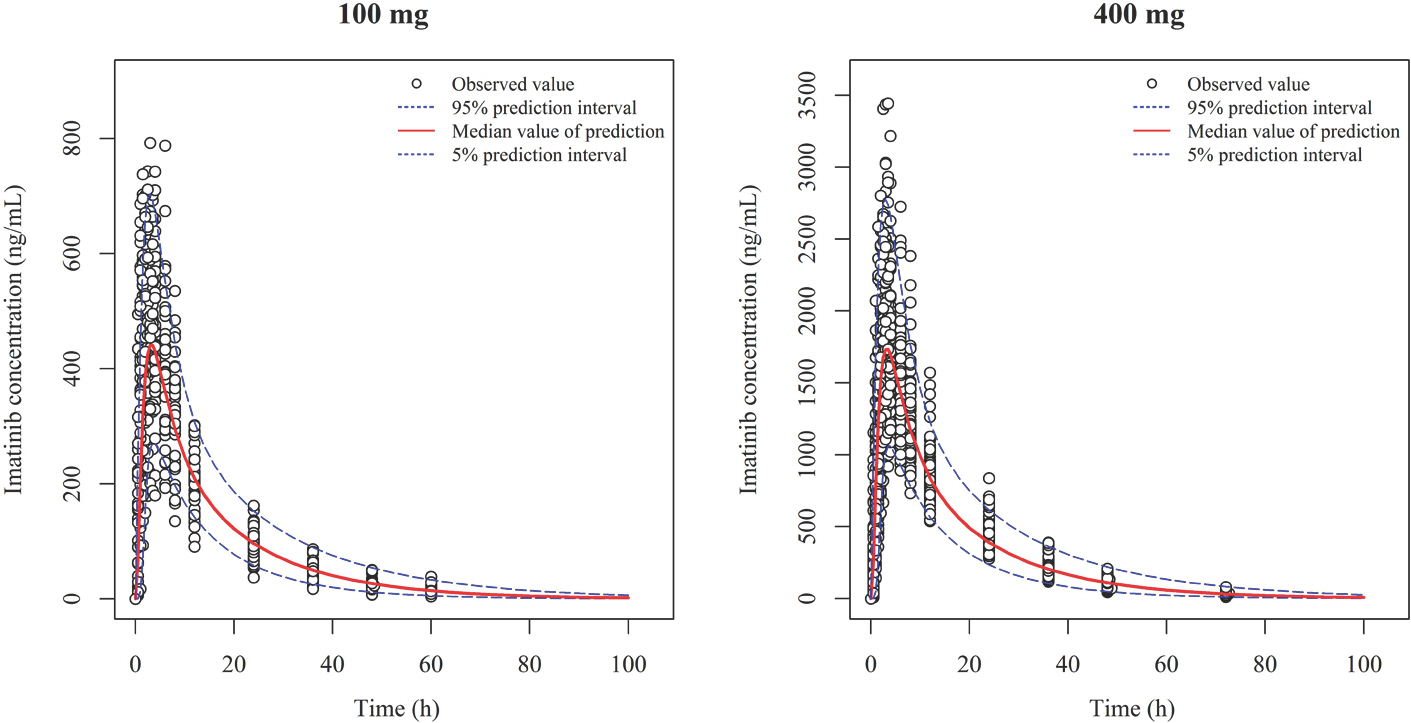
- Imatinib (Gleevecâ„¢; Novartis Pharmaceuticals) is an orally administered protein-tyrosine kinase inhibitor. The goal of this study was to investigate the population pharmacokinetics (PK) of imatinib (as imatinib mesylate) in healthy male Koreans. A total of 1,773 plasma samples from 112 healthy male volunteers enrolled in three phase I clinical studies were used. Among the subjects, 76 received 400 mg and 36 received 100 mg as single oral doses. Peripheral blood sampling for PK analysis was done at 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 6, 8, 12, 24, 48, 60 and 72 (at 400 mg group) h after dosing. The firstorder conditional estimation with interaction method of NONMEM® (ver. 7.3) was used to build the population PK model. A two-compartment model with Weibull absorption and elimination gave the best fit to the data. The estimates of clearance (CL/F), volume of central compartment (Vc/F), intercompartmental clearance (Q/F), peripheral volume (Vp/F) and their interindividual variabily (%CV) were 13.6 L/h (23.4%), 153 L (29.2%), 8.64 L/h (35.9%) and 64 L (67%), respectively.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Arora A, Scholar EM. Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther. 2005; 315:971–979.
Article2. Paul MK, Mukhopadhyay AK. Tyrosine kinase – Role and significance in Cancer. Int J Med Sci. 2004; 1:101–115.
Article3. Druker BJ. Imatinib as a paradigm of targeted therapies. Adv Cancer Res. 2004; 91:1–30.
Article4. Savage DG, Antman KH. Imatinib mesylate–a new oral targeted therapy. N Engl J Med. 2002; 346:683–693.5. Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non–small-cell lung cancer: Current knowledge and future directions. J Clin Oncol. 2005; 23:2556–2568.
Article6. Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002; 100:1532–1542.
Article7. Sharma A, Trivedi NR, Zimmerman MA, Tuveson DA, Smith CD, Robertson GP. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005; 65:2412–2421.
Article8. Buchdunger E, Zimmermann J, Mett H, Meyer T, Müller M, Druker BJ, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 1996; 56:100–104.9. Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000; 96:925–932.
Article10. Cohen MH, Williams G, Johnson JR, Duan J, Gobburu J, Rahman A, et al. Approval Summary for Imatinib Mesylate Capsules in the Treatment of Chronic Myelogenous Leukemia. Clin Cancer Res. 2002; 8:935–942.11. Dagher R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res. 2002; 8:3034–3038.12. Capdeville R, Silberman S, Dimitrijevic S. Imatinib: the first 3 years. Eur J Cancer. 2002; 38:S77–S82.
Article13. Petain A, Kattygnarath D, Azard J, Chatelut E, Delbaldo C, Geoerger B, et al. Population pharmacokinetics and pharmacogenetics of imatinib in children and adults. Clin Cancer Res. 2008; 14:7102–7109.
Article14. Widmer N, Decosterd LA, Csajka C, Leyvraz S, Duchosal MA, Rosselet A, et al. Population pharmacokinetics of imatinib and the role of α1-acid glycoprotein. Br J Clin Pharmacol. 2006; 62:97–112.15. Schmidli H, Peng B, Riviere GJ, Capdeville R, Hensley M, Gathmann I, et al. Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol. 2005; 60:35–44.
Article16. Titier K, Picard S, Ducint D, Teilhet E, Moore N, Berthaud P, et al. Quantification of imatinib in human plasma by high-performance liquid chromatography-tandem mass spectrometry. Ther Drug Monit. 2005; 27:634–640.
Article17. Parise RA, Ramanathan RK, Hayes MJ, Egorin MJ. Liquid chromatographic-mass spectrometric assay for quantitation of imatinib and its main metabolite (CGP 74588) in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 791:39–44.
Article18. Team RC. R: A language and environment for statistical computing. 2015.19. Jonsson EN, Karlsson MO. Xpose–an S-PLUS based population phar-macokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1998; 58:51–64.
Article20. Zhou H. Pharmacokinetic strategies in deciphering atypical drug absorption profiles. J Clin Pharmacol. 2003; 43:211–227.
Article21. Hastie T. gam: Generalized additive models. https://cran.r-project.org/web/packages/gam/gam.pdf. Accessed May 1. 2016.22. Holford N. Wings for NONMEM. 2016. http://wfn.sourceforge.net/AccessedJan10.23. Delbaldo C, Chatelut E, Ré M, Deroussent A, Séronie-Vivien S, Jambu A, et al. Pharmacokinetic-pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res. 2006; 12:6073–6078.
Article24. Judson I, Ma P, Peng B, Verweij J, Racine A, di Paola ED, et al. Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol. 2005; 55:379–386.
Article25. Menon-Andersen D, Mondick JT, Jayaraman B, Thompson PA, Blaney SM, Bernstein M, et al. Population pharmacokinetics of imatinib mesylate and its metabolite in children and young adults. Cancer Chemother Pharmacol. 2009; 63:229–238.
Article26. Yamakawa Y, Hamada A, Nakashima R, Yuki M, Hirayama C, Kawaguchi T, et al. Association of genetic polymorphisms in the influx transporter SL-CO1B3 and the efflux transporter ABCB1 with imatinib pharmacokinetics in patients with chronic myeloid leukemia. Ther Drug Monit. 2011; 33:244–250.27. Demetri GD, Wang Y, Wehrle E, Racine A, Nikolova Z, Blanke CD, et al. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. J Clin Oncol. 2009; 27:3141–3147.
Article28. Piotrovskii VK. The use of Weibull distribution to describe thein vivo absorption kinetics. J Pharmacokinet Biopharm. 1987; 15:681–686.
Article29. Piotrovskii VK. Pharmacokinetic stochastic model with Weibull-distributed residence times of drug molecules in the body. Eur J Clin Pharmacol. 1987; 32:515–523.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Lichenoid Drug Eruption Associated with Imatinib Mesylate
- Exacerbation of Psoriasis after Imatinib Mesylate Treatment
- A Case of Generalized Keratosis Pilaris Induced by Imatinib Mesylate
- Loeffler endocarditis in chronic eosinophilic leukemia with FIP1L1/PDGFRA rearrangement: full recovery with low dose imatinib
- Imatinib Mesylate-Induced Hyperpigmentation of the Nose and Palate