J Gynecol Oncol.
2013 Apr;24(2):160-166. 10.3802/jgo.2013.24.2.160.
Comparison of advanced stage mucinous epithelial ovarian cancer and serous epithelial ovarian cancer with regard to chemosensitivity and survival outcome: a matched case-control study
- Affiliations
-
- 1Department of Gynecologic Oncology, Etlik Zubeyde Hanim Women's Health Teaching and Research Hospital, Ankara, Turkey.
- 2Department of Obstetrics and Gynecology, Bahcesehir University Faculty of Medicine, Istanbul, Turkey.
- 3Department of Obstetrics and Gynecology, Fatih University Faculty of Medicine, Ankara, Turkey. denizhizli@yahoo.com
- 4Department of Gynecologic Oncology, Ankara University Faculty of Medicine, Ankara, Turkey.
- 5Department of Pathology, Etlik Zubeyde Hanim Women's Health Teaching and Research Hospital, Ankara, Turkey.
- KMID: 2288548
- DOI: http://doi.org/10.3802/jgo.2013.24.2.160
Abstract
OBJECTIVE
The aim of this study was to compare clinicopathologic characteristics, surgery outcomes and survival outcomes of patients with stage III and IV mucinous epithelial ovarian cancer (mEOC) and serous epithelial ovarian carcinoma (sEOC).
METHODS
Patients who had surgery for advanced stage (III or IV) mEOC were evaluated retrospectively and defined as the study group. Women with sEOC who were matched for age and stage of disease were randomly chosen from the database and defined as the control group. The baseline disease characteristics of patients and platinum-based chemotherapy efficacy (response rate, progression-free survival and overall survival [OS]) were compared.
RESULTS
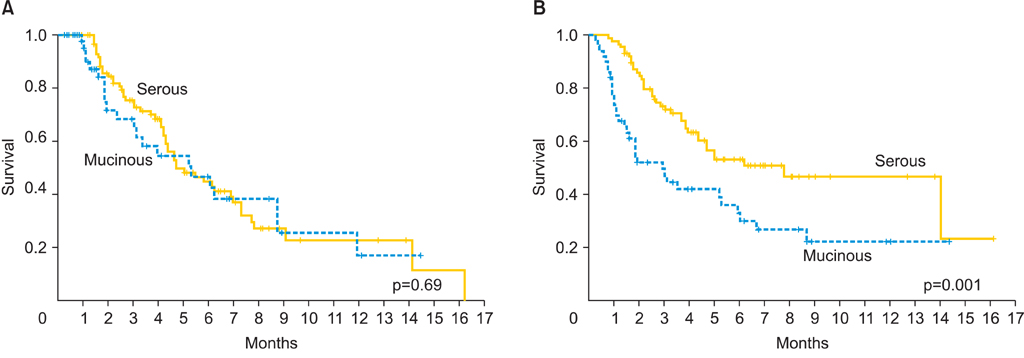
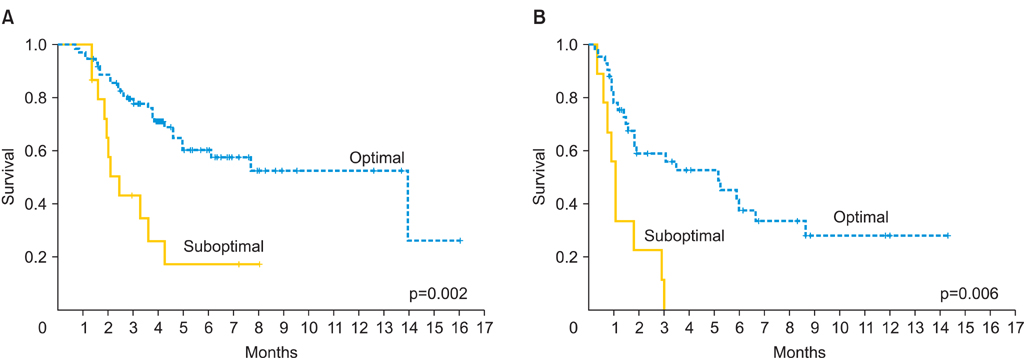
A total of 138 women were included in the study: 50 women in the mEOC group and 88 in the sEOC group. Patients in the mEOC group had significantly less grade 3 tumors and CA-125 levels and higher rate of para-aortic and pelvic lymph node metastasis. Patients in the mEOC group had significantly less platinum sensitive disease (57.9% vs. 70.8%; p=0.03) and had significantly poorer OS outcome when compared to the sEOC group (p=0.001). The risk of death for mEOC patients was significantly higher than for sEOC patients (hazard ratio, 2.14; 95% confidence interval, 1.34 to 3.42).
CONCLUSION
Advanced stage mEOC patients have more platinum resistance disease and poorer survival outcome when compared to advanced stage sEOC. Therefore, novel chemotherapy strategies are warranted to improve survival outcome in patients with mEOC.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Safety of Fertility-Sparing Surgery in Primary Mucinous Carcinoma of the Ovary
Jung-Yun Lee, Yu Ri Jo, Tae Hun Kim, Hee Seung Kim, Min A Kim, Jae Weon Kim, Noh Hyun Park, Yong-Sang Song
Cancer Res Treat. 2015;47(2):290-297. doi: 10.4143/crt.2014.004.
Reference
-
1. Zang RY, Li ZT, Tang J, Cheng X, Cai SM, Zhang ZY, et al. Secondary cytoreductive surgery for patients with relapsed epithelial ovarian carcinoma: who benefits? Cancer. 2004. 100:1152–1161.2. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996. 334:1–6.3. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002. 20:1248–1259.4. Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008. 109:370–376.5. Omura GA, Brady MF, Homesley HD, Yordan E, Major FJ, Buchsbaum HJ, et al. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology Group experience. J Clin Oncol. 1991. 9:1138–1150.6. Teramukai S, Ochiai K, Tada H, Fukushima M. PIEPOC: a new prognostic index for advanced epithelial ovarian cancer: Japan Multinational Trial Organization OC01-01. J Clin Oncol. 2007. 25:3302–3306.7. Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal: a Gynecologic Oncology Group study. Cancer. 2011. 117:554–562.8. Schiavone MB, Herzog TJ, Lewin SN, Deutsch I, Sun X, Burke WM, et al. Natural history and outcome of mucinous carcinoma of the ovary. Am J Obstet Gynecol. 2011. 205:480.e1–480.e8.9. Alexandre J, Ray-Coquard I, Selle F, Floquet A, Cottu P, Weber B, et al. Mucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experience. Ann Oncol. 2010. 21:2377–2381.10. Nakayama K, Takebayashi Y, Nakayama S, Hata K, Fujiwaki R, Fukumoto M, et al. Prognostic value of overexpression of p53 in human ovarian carcinoma patients receiving cisplatin. Cancer Lett. 2003. 192:227–235.11. Shimada M, Kigawa J, Ohishi Y, Yasuda M, Suzuki M, Hiura M, et al. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2009. 113:331–334.12. Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975. 42:101–104.13. Hess V, A'Hern R, Nasiri N, King DM, Blake PR, Barton DP, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004. 22:1040–1044.14. Bamias A, Psaltopoulou T, Sotiropoulou M, Haidopoulos D, Lianos E, Bournakis E, et al. Mucinous but not clear cell histology is associated with inferior survival in patients with advanced stage ovarian carcinoma treated with platinum-paclitaxel chemotherapy. Cancer. 2010. 116:1462–1468.15. Mackay HJ, Brady MF, Oza AM, Reuss A, Pujade-Lauraine E, Swart AM, et al. Prognostic relevance of uncommon ovarian histology in women with stage III/IV epithelial ovarian cancer. Int J Gynecol Cancer. 2010. 20:945–952.16. Pectasides D, Fountzilas G, Aravantinos G, Kalofonos HP, Efstathiou E, Salamalekis E, et al. Advanced stage mucinous epithelial ovarian cancer: the Hellenic Cooperative Oncology Group experience. Gynecol Oncol. 2005. 97:436–441.17. Pignata S, Ferrandina G, Scarfone G, Scollo P, Odicino F, Cormio G, et al. Activity of chemotherapy in mucinous ovarian cancer with a recurrence free interval of more than 6 months: results from the SOCRATES retrospective study. BMC Cancer. 2008. 8:252.18. Cheng X, Jiang R, Li ZT, Tang J, Cai SM, Zhang ZY, et al. The role of secondary cytoreductive surgery for recurrent mucinous epithelial ovarian cancer (mEOC). Eur J Surg Oncol. 2009. 35:1105–1108.19. Tian C, Markman M, Zaino R, Ozols RF, McGuire WP, Muggia FM, et al. CA-125 change after chemotherapy in prediction of treatment outcome among advanced mucinous and clear cell epithelial ovarian cancers: a Gynecologic Oncology Group study. Cancer. 2009. 115:1395–1403.20. Trimble EL, Birrer MJ, Hoskins WJ, Marth C, Petryshyn R, Quinn M, et al. Current academic clinical trials in ovarian cancer: Gynecologic Cancer Intergroup and US National Cancer Institute Clinical Trials Planning Meeting, May 2009. Int J Gynecol Cancer. 2010. 20:1290–1298.21. Shimizu Y, Nagata H, Kikuchi Y, Umezawa S, Hasumi K, Yokokura T. Cytotoxic agents active against mucinous adenocarcinoma of the ovary. Oncol Rep. 1998. 5:99–101.22. Shimizu Y, Umezawa S, Hasumi K. A phase II study of combined CPT-11 and mitomycin-C in platinum refractory clear cell and mucinous ovarian carcinoma. Ann Acad Med Singapore. 1998. 27:650–656.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Significance of Serum CA 125 Levels in Patients with Advanced Serous Epithelial Ovarian Cancer
- A Case of Recurrent Early-stage Epithelial Ovarian Cancer Presenting as Bone Metastasis
- Ovarian malignancy in pregnancy: Experience for 17 years
- P21 and p53 expression in patients with epithelial ovarian carcinoma
- An Analysis of Clinicopathologic Prognostic Factors affecting Survival in Patients with Epithelial Ovarian Cancer