J Clin Neurol.
2013 Jul;9(3):157-164. 10.3988/jcn.2013.9.3.157.
Effect of the Refrigerator Storage Time on the Potency of Botox for Human Extensor Digitorum Brevis Muscle Paralysis
- Affiliations
-
- 1Department of Neurology, College of Medicine, Yeungnam University, Daegu, Korea. mypark@med.yu.ac.kr
- 2Ahn's Plastic and Reconstructive Surgery Clinic and Botulinum Center, Daegu, Korea.
- KMID: 2287554
- DOI: http://doi.org/10.3988/jcn.2013.9.3.157
Abstract
- BACKGROUND AND PURPOSE
It is recommended that Botox be used within 5 hours of reconstitution, which results in substantial quantities being discarded. This is not only uneconomic, but also inconvenient for treating patients. The aim of this study was to determine the potencies of Botox used within 2 hours of reconstitution with unpreserved saline, the same Botox refrigerated (at +4degrees C) 72 hours after reconstitution, and during the next 4 consecutive weeks (weeks 1, 2, 3, and 4). This comparison was used to determine the length of refrigeration time during which reconstituted Botox will maintain the same efficacy as freshly reconstituted toxin.
METHODS
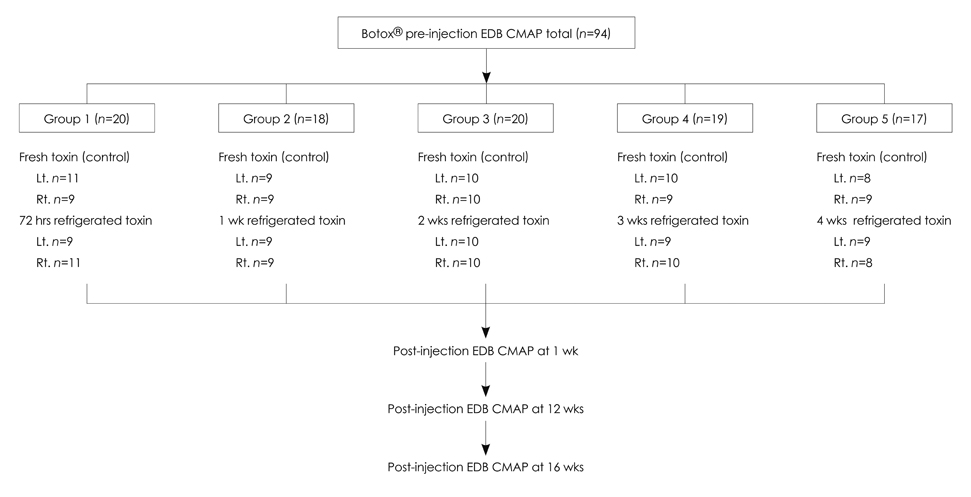
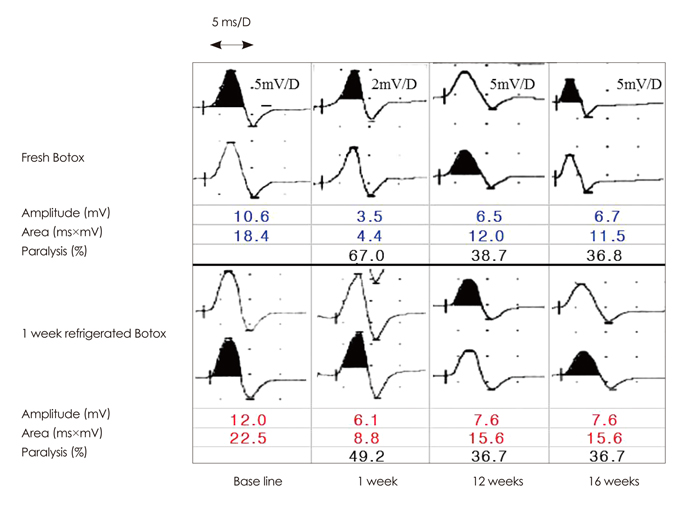
Individual paralysis rates in the extensor digitorum brevis (EDB) compound muscle action potential (CMAP) amplitude and area were measured 1 week after injecting fresh reconstituted 2.5 MU of Botox on one side of the foot, and when the same quantity of Botox that had been refrigerated for a designated time (i.e., 72 h, or 1, 2, 3, or 4 weeks) into the other side of the foot. The EDB CMAP amplitude and area at 12 and 16 weeks postinjection were also measured to compare the efficacy durations in all five comparative groups.
RESULTS
Ninety-four volunteers were divided into five groups according to the refrigerator storage time of the second Botox injection. The paralysis of the EDBs was significant for each injection of Botox, both fresh and refrigerated, with no statistically significant differences between them, regardless of the refrigeration time. There was a tendency toward increased CMAP amplitude and area at 12 or 16 weeks postinjection (p<0.0001). The duration of effective muscle paralysis did not differ significantly throughout the 16-week follow-up period between all five groups.
CONCLUSIONS
The potency of reconstituted Botox is not degraded by subsequent refrigeration for 4 weeks. However, there are definite concerns regarding its sterility, and hence its safety, since multiple withdrawals from the same vial over long periods can introduce bacterial contamination.
Keyword
MeSH Terms
Figure
Reference
-
1. Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993; 365:160–163.
Article2. Schiavo G, Santucci A, Dasgupta BR, Mehta PP, Jontes J, Benfenati F, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993; 335:99–103.
Article3. Duchen LW. An electron microscopic study of the changes induced by botulinum toxin in the motor end-plates of slow and fast skeletal muscle fibres of the mouse. J Neurol Sci. 1971; 14:47–60.
Article4. Molgo J, Comella JX, Angaut-Petit D, Pecot-Dechavassine M, Tabti N, Faille L, et al. Presynaptic actions of botulinal neurotoxins at vertebrate neuromuscular junctions. J Physiol (Paris). 1990; 84:152–166.5. Brin MF. Botulinum toxin: new and expanded indications. Eur J Neurol. 1997; 4:S59–S65.6. Ahn KY, Park MY, Park DH, Han DG. Botulinum toxin A for the treatment of facial hyperkinetic wrinkle lines in Koreans. Plast Reconstr Surg. 2000; 105:778–784.
Article7. Carruthers J, Carruthers A. Botulinum toxin (botox) chemodenervation for facial rejuvenation. Facial Plast Surg Clin North Am. 2001; 9:197–204. vii8. Park MY, Ahn KY, Jung DS. Botulinum toxin type A treatment for contouring of the lower face. Dermatol Surg. 2003; 29:477–483. discussion 483.
Article9. Brin MF, Binder WJ, Blitzer A, Schenrock L, Pogoda JM. Botulinum toxin type A for pain and headache. In : Brin MF, Hallett M, Jankovic J, editors. Scientific and Therapeutic Aspects of Botulinum Toxin. Philadelphia, PA: Lippincott Williams & Wilkins;2002.10. Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001; 323:596–599.
Article11. Klein AW. Dilution and storage of botulinum toxin. Dermatol Surg. 1998; 24:1179–1180.
Article12. Gartlan MG, Hoffman HT. Crystalline preparation of botulinum toxin type A (Botox): degradation in potency with storage. Otolaryngol Head Neck Surg. 1993; 108:135–140.
Article13. Sloop RR, Cole BA, Escutin RO. Human response to botulinum toxin injection: type B compared with type A. Neurology. 1997; 49:189–194.
Article14. Paik NJ, Seo K, Eun HC. Reduced potency after refrigerated storage of botulitum toxin A: human extensor digitorum brevis muscle study. Mov Disord. 2006; 21:1759–1763.
Article15. Sloop RR, Cole BA, Escutin RO. Reconstituted botulinum toxin type A does not lose potency in humans if it is refrozen or refrigerated for 2 weeks before use. Neurology. 1997; 48:249–253.
Article16. Yang GC, Chiu RJ, Gillman GS. Questioning the need to use Botox within 4 hours of reconstitution: a study of fresh vs 2-week-old Botox. Arch Facial Plast Surg. 2008; 10:273–279.17. Garcia A, Fulton JE Jr. Cosmetic denervation of the muscles of facial expression with botulinum toxin. A dose-response study. Dermatol Surg. 1996; 22:39–43.
Article18. Hexsel DM, De Almeida AT, Rutowitsch M, De Castro IA, Silveira VL, Gobatto DO, et al. Multicenter, double-blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before application. Dermatol Surg. 2003; 29:523–529. discussion 529.
Article19. Dolman CE. Botulism as a world problem. In : Lewis KH, Cassel K, editors. Botulism. Cincinnati, OH: U.S. Dept. of Health, Education, and Welfare, Public Health Service;1964.20. Hambleton P. Clostridium botulinum toxins: a general review of involvement in disease, structure, mode of action and preparation for clinical use. J Neurol. 1992; 239:16–20.
Article21. Jessell TM, Kandel ER. Synaptic transmission: a bidirectional and self-modifiable form of cell-cell communication. Cell. 1993; 72:Suppl. 1–30.
Article22. Montecucco C, Schiavo G. Tetanus and botulism neurotoxins: a new group of zinc proteases. Trends Biochem Sci. 1993; 18:324–327.
Article23. Schiavo G, Rossetto O, Benfenati F, Poulain B, Montecucco C. Tetanus and botulinum neurotoxins are zinc proteases specific for components of the neuroexocytosis apparatus. Ann N Y Acad Sci. 1994; 710:65–75.
Article24. Shaari CM, Sanders I. Quantifying how location and dose of botulinum toxin injections affect muscle paralysis. Muscle Nerve. 1993; 16:964–969.
Article25. Borodic GE, Cozzolino D, Ferrante R, Wiegner AW, Young RR. Innervation zone of orbicularis oculi muscle and implications for botulinum A toxin therapy. Ophthal Plast Reconstr Surg. 1991; 7:54–60.
Article26. Borodic GE, Pearce LB, Smith K, Joseph M. Botulinum a toxin for spasmodic torticollis: multiple vs single injection points per muscle. Head Neck. 1992; 14:33–37.
Article27. Hambleton P, Cohen HE, Palmer BJ, Melling J. Antitoxins and botulinum toxin treatment. BMJ. 1992; 304:959–960.
Article28. Zuber M, Sebald M, Bathien N, de Recondo J, Rondot P. Botulinum antibodies in dystonic patients treated with type A botulinum toxin: frequency and significance. Neurology. 1993; 43:1715–1718.
Article29. Greene P. Potency of frozen/thawed botulinum toxin type A in the treatment of torsion dystonia. Otolaryngol Head Neck Surg. 1993; 109:968–969.
Article30. First ER, Pearce LB, Borodic GE. Dose standardisation of botulinum toxin. Lancet. 1994; 343:1035.
Article31. Pickett AM, Hambleton P. Dose standardisation of botulinum toxin. Lancet. 1994; 344:474–475.
Article32. Hughes R, Whaler BC. Influence of nerve-ending activity and of drugs on the rate of paralysis of rat diaphragm preparations by Cl. botulinum type A toxin. J Physiol. 1962; 160:221–233.
Article33. Eleopra R, Tugnoli V, De Grandis D. The variability in the clinical effect induced by botulinum toxin type A: the role of muscle activity in humans. Mov Disord. 1997; 12:89–94.
Article34. Kim HS, Hwang JH, Jeong ST, Lee YT, Lee PK, Suh YL, et al. Effect of muscle activity and botulinum toxin dilution volume on muscle paralysis. Dev Med Child Neurol. 2003; 45:200–206.
Article35. Glocker FX, Guschlbauer B, Lücking CH, Deuschl G. Effects of local injections of botulinum toxin on electrophysiological parameters in patients with hemifacial spasm: role of synaptic activity and size of motor units. Neurosci Lett. 1995; 187:161–164.
Article36. Hesse S, Jahnke MT, Luecke D, Mauritz KH. Short-term electrical stimulation enhances the effectiveness of Botulinum toxin in the treatment of lower limb spasticity in hemiparetic patients. Neurosci Lett. 1995; 201:37–40.
Article37. Holds JB, Fogg SG, Anderson RL. Botulinum A toxin injection. Failures in clinical practice and a biomechanical system for the study of toxin-induced paralysis. Ophthal Plast Reconstr Surg. 1990; 6:252–259.
Article38. Sloop RR, Escutin RO, Matus JA, Cole BA, Peterson GW. Dose-response curve of human extensor digitorum brevis muscle function to intramuscularly injected botulinum toxin type A. Neurology. 1996; 46:1382–1386.
Article39. Kauffman JA, Way JF Jr, Siegel LS, Sellin LC. Comparison of the action of types A and F botulinum toxin at the rat neuromuscular junction. Toxicol Appl Pharmacol. 1985; 79:211–217.
Article40. Frueh BR, Felt DP, Wojno TH, Musch DC. Treatment of blepharospasm with botulinum toxin. A preliminary report. Arch Ophthalmol. 1984; 102:1464–1468.41. Borodic GE, Cozzolino D. Blepharospasm and its treatment, with emphasis on the use of botulinum toxin. Plast Reconstr Surg. 1989; 83:546–554.
Article42. Pearce LB, Borodic GE, First ER, MacCallum RD. Measurement of botulinum toxin activity: evaluation of the lethality assay. Toxicol Appl Pharmacol. 1994; 128:69–77.
Article43. Pearce LB, Borodic GE, Johnson EA, First ER, MacCallum R. The median paralysis unit: a more pharmacologically relevant unit of biologic activity for botulinum toxin. Toxicon. 1995; 33:217–227.
Article44. Kessler KR, Benecke R. The EBD test--a clinical test for the detection of antibodies to botulinum toxin type A. Mov Disord. 1997; 12:95–99.
Article45. Gordon PH, Gooch CL, Greene PE. Extensor digitorum brevis test and resistance to botulinum toxin type A. Muscle Nerve. 2002; 26:828–831.
Article46. Houser MK, Sheean GL, Lees AJ. Further studies using higher doses of botulinum toxin type F for torticollis resistant to botulinum toxin type A. J Neurol Neurosurg Psychiatry. 1998; 64:577–580.
Article47. Inagi K, Schultz E, Ford CN. An anatomic study of the rat larynx: establishing the rat model for neuromuscular function. Otolaryngol Head Neck Surg. 1998; 118:74–81.
Article48. Hamjian JA, Walker FO. Serial neurophysiological studies of intramuscular botulinum-A toxin in humans. Muscle Nerve. 1994; 17:1385–1392.
Article49. Park MY, Ahn KY. Botulinum toxin a for the treatment of hyperkinetic wrinkle lines. Plast Reconstr Surg. 2003; 112:5 Suppl. 148S–150S.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Muscular Variations of Extensor Digitorum Brevis Muscle Related with Anterior Tarsal Tunnel Syndrome
- An Extensor Digitorum Muscle for Index Finger Originated from the Extensor Carpi Radialis Brevis
- Extensor Digitorum Brevis Manus
- Extensor Digitorum Brevis Innervated by the Tibial Nerve (All Tibial Foot): A case report
- Effect of Storage Temperature and Durationon Potency of Botulinum Toxin