J Clin Neurol.
2013 Jul;9(3):151-156. 10.3988/jcn.2013.9.3.151.
A Pilot Study of Fluorodeoxyglucose Positron Emission Tomography Findings in Patients with Phenylketonuria before and during Sapropterin Supplementation
- Affiliations
-
- 1Children's Hospital of Philadelphia, University of Pennsylvania, Perelman School of Medicine, Section of Biochemical Genetics, Philadelphia, PA, USA. ficicioglu@email.chop.edu
- 2Division of Nuclear Medicine and Clinical Molecular Imaging, Department of Radiology, Philadelphia, PA, USA.
- 3Department of Child and Adolescent Psychiatry and Behavioral Sciences, Philadelphia, PA, USA.
- 4Biostatistics Core, The Clinical and Translational Research Center, Philadelphia, PA, USA.
- KMID: 2287553
- DOI: http://doi.org/10.3988/jcn.2013.9.3.151
Abstract
- BACKGROUND AND PURPOSE
PET scanning with fluorodeoxyglucose (FDG-PET) is a non-invasive method that measures regional glucose metabolic rate. Phenylalanine (Phe) and its metabolites appear to impair several aspects of brain energy metabolism. 1) To evaluate brain glucose metabolism with FDG-PET imaging in phenylketonuria (PKU) patients before and 4 months after sapropterin therapy; 2) to evaluate neurodevelopmental changes, blood Phe levels and dietary Phe tolerance before and after sapropterin therapy; 3) to generate pilot data to assess the feasibility of evaluating brain glucose metabolism with FDG-PET imaging and to explore potential trends resulting from the administration of sapropterin therapy.
METHODS
We enrolled 5 subjects, ranged in age from 22 years to 51 years, with PKU. Subjects underwent FDG-PET brain imaging, blood tests for Phe and tyrosine levels, and neurocognitive evaluations before and 4 months after sapropterin therapy (20 mg/kg/day). All subjects' Phe and tyrosine levels were monitored once a week during the study. Subjects kept 3 day diet records that allow calculation of Phe intake.
RESULTS
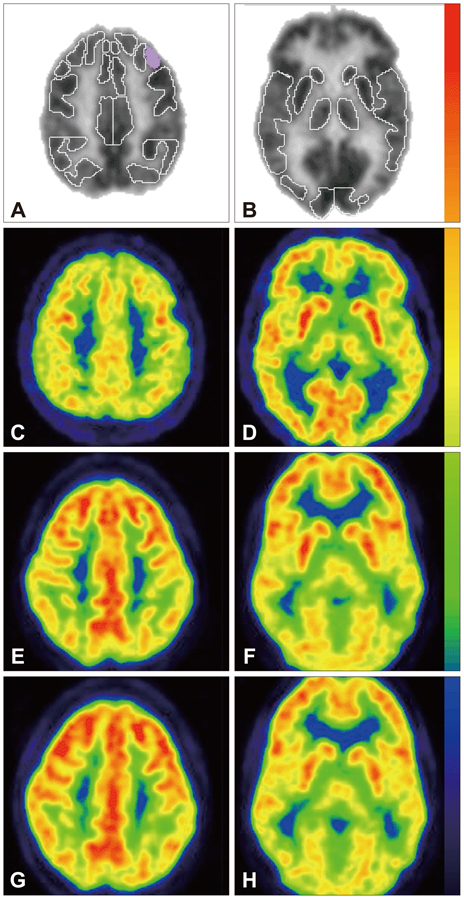
None of the subjects responded to sapropterin therapy based on 30% decrease in blood Phe level. The data show that glucose metabolism appeared depressed in the cerebellum and left parietal cortex while it was increased in the frontal and anterior cingulate cortices in all five subjects. In response to sapropterin therapy, relative glucose metabolism showed significant increases in left Broca's and right superior lateral temporal cortices. Interestingly, there was corresponding enhanced performance in a phonemic fluency test performed during pre- and postneurocognitive evaluation.
CONCLUSIONS
Further studies with a larger sample size are needed to confirm the above changes in both sapropterin non-responsive and responsive PKU patients.
MeSH Terms
Figure
Reference
-
1. Scriver CR, Kaufman S. Hyperphenylalaninemia: phenylalanine hydroxylase deficiency. In : Scriver CR, Beaudet AL, Valle D, Sly WS, Childs B, Kinzler KW, editors. The Metabolic and Molecular Bases of Inherited Disease. Vol 4:8th ed. New York: McGraw-Hill;2001. p. 1667–1709.2. Waisbren SE, Noel K, Fahrbach K, Cella C, Frame D, Dorenbaum A, et al. Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol Genet Metab. 2007; 92:63–70.
Article3. de Groot MJ, Hoeksma M, Blau N, Reijngoud DJ, van Spronsen FJ. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010; 99:Suppl 1. S86–S89.
Article4. Landvogt C, Mengel E, Bartenstein P, Buchholz HG, Schreckenberger M, Siessmeier T, et al. Reduced cerebral fluoro-L-dopamine uptake in adult patients suffering from phenylketonuria. J Cereb Blood Flow Metab. 2008; 28:824–831.
Article5. Rech VC, Feksa LR, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM. Inhibition of the mitochondrial respiratory chain by phenylalanine in rat cerebral cortex. Neurochem Res. 2002; 27:353–357.6. Pietz J, Rupp A, Ebinger F, Rating D, Mayatepek E, Boesch C, et al. Cerebral energy metabolism in phenylketonuria: findings by quantitative In vivo 31P MR spectroscopy. Pediatr Res. 2003; 53:654–662.
Article7. Wasserstein MP, Snyderman SE, Sansaricq C, Buchsbaum MS. Cerebral glucose metabolism in adults with early treated classic phenylketonuria. Mol Genet Metab. 2006; 87:272–277.
Article8. Hennermann JB, Bührer C, Blau N, Vetter B, Mönch E. Long-term treatment with tetrahydrobiopterin increases phenylalanine tolerance in children with severe phenotype of phenylketonuria. Mol Genet Metab. 2005; 86:Suppl 1. S86–S90.
Article9. Matalon R, Michals-Matalon K, Koch R, Grady J, Tyring S, Stevens RC. Response of patients with phenylketonuria in the US to tetrahydrobiopterin. Mol Genet Metab. 2005; 86:Suppl 1. S17–S21.
Article10. Muntau AC, Röschinger W, Habich M, Demmelmair H, Hoffmann B, Sommerhoff CP, et al. Tetrahydrobiopterin as an alternative treatment for mild phenylketonuria. N Engl J Med. 2002; 347:2122–2132.
Article11. Alavi A, Reivich M, Ferris S, Christman D, Fowler J, MacGregor R, et al. Regional cerebral glucose metabolism in aging and senile dementia as determined by 18F-deoxyglucose and positron emission tomography. Exp Brain Res. 1982; Suppl 5. 187–195.
Article12. Sestini S, Castagnoli A, Mansi L. The new FDG brain revolution: the neurovascular unit and the default network. Eur J Nucl Med Mol Imaging. 2010; 37:913–916.
Article13. Koshimura K, Miwa S, Lee K, Fujiwara M, Watanabe Y. Enhancement of dopamine release in vivo from the rat striatum by dialytic perfusion of 6R-L-erythro-5,6,7,8-tetrahydrobiopterin. J Neurochem. 1990; 54:1391–1397.
Article14. Mataga N, Imamura K, Watanabe Y. 6R-tetrahydrobiopterin perfusion enhances dopamine, serotonin, and glutamate outputs in dialysate from rat striatum and frontal cortex. Brain Res. 1991; 551:64–71.
Article15. Koshimura K, Miwa S, Watanabe Y. Dopamine-releasing action of 6R-L-erythro-tetrahydrobiopterin: analysis of its action site using sepiapterin. J Neurochem. 1994; 63:649–654.
Article16. Kim HL, Park YS. Maintenance of cellular tetrahydrobiopterin homeostasis. BMB Rep. 2010; 43:584–592.
Article17. Sanayama Y, Nagasaka H, Takayanagi M, Ohura T, Sakamoto O, Ito T, et al. Experimental evidence that phenylalanine is strongly associated to oxidative stress in adolescents and adults with phenylketonuria. Mol Genet Metab. 2011; 103:220–225.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transient ¹â¸F-Fluorodeoxyglucose Activity on PET/CT of Herniation Pit in Thyroid Cancer Patient: A Case Report
- Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Findings of Post Traumatic Lymphangioma in a Young Adult Male
- F-18 fluorodeoxyglucose positron emission tomography/computed tomography in the infection of heart
- The importance of 18F-fluorodeoxyglucose positron emission tomography and carbohydrate antigen 19-9 in patients with periampullary tumors
- Non-Malignant 18F-FDG Uptake in the Thorax by Positron Emission Tomography Computed Tomography Fusion Imaging