J Breast Cancer.
2012 Dec;15(4):401-406. 10.4048/jbc.2012.15.4.401.
Analysis of the Potent Prognostic Factors in Luminal-Type Breast Cancer
- Affiliations
-
- 1Department of Surgery, Inje University Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea. s2606@inje.ac.kr
- KMID: 2286436
- DOI: http://doi.org/10.4048/jbc.2012.15.4.401
Abstract
- PURPOSE
Luminal-type breast cancer has a good prognosis compared to other types, such as human epidermal growth factor receptor 2 and triple negative types. Luminal-type breast cancer is classified into luminal A and B, according to the proliferation index. We investigated the clinicopathological factors that affect the prognosis of the luminal-type subgroups.
METHODS
We reviewed the medical records and the pathologic reports of 159 luminal-type breast cancer patients who were treated between February 2005 and November 2007. We divided luminal-type breast cancer into luminal A and B, according to Ki-67 (cutoff value, 14%) and analyzed the clinicopathologic factors, such as age at diagnosis, intensity score of estrogen receptor and progesterone receptor, histologic grade, and Bcl-2. Moreover, we compared the disease-free survival (DFS) of each group.
RESULTS
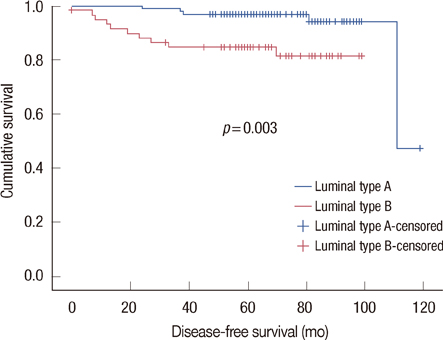
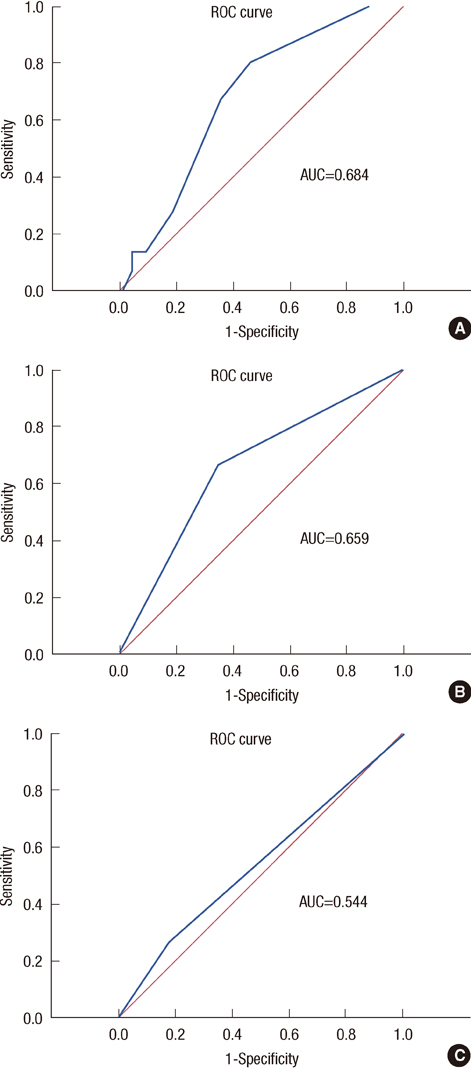
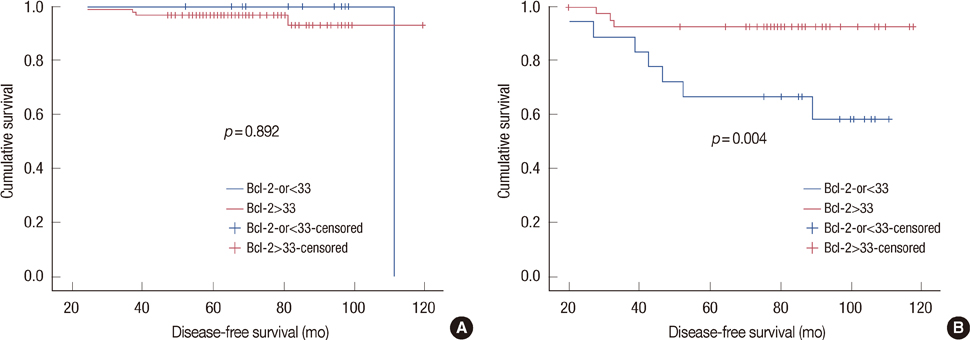
In the univariate analysis, age (p=0.004), tumor size (p=0.010), lymph node metastasis (p=0.001), and Bcl-2 (p=0.002) were statistically significant factors in luminal-type breast cancer. In the multivariate analysis, lymph node (p=0.049) and Bcl-2 (p=0.034) were significant relevant factors in luminal-type breast cancer. In the subgroup analysis, the increased Bcl-2 (cutoff value, 33%) was related with a longer DFS in the luminal B group (p=0.004).
CONCLUSION
In our study, luminal A breast cancer showed a longer DFS than luminal B breast cancer, further, Bcl-2 may be a potent prognostic factor in luminal-type breast cancer.
Keyword
MeSH Terms
-
Breast
Breast Neoplasms
Disease-Free Survival
Estrogens
Humans
Lymph Nodes
Medical Records
Multivariate Analysis
Neoplasm Metastasis
Phenobarbital
Prognosis
Receptor, Epidermal Growth Factor
Receptor, erbB-2
Receptors, Progesterone
Estrogens
Phenobarbital
Receptor, Epidermal Growth Factor
Receptor, erbB-2
Receptors, Progesterone
Figure
Cited by 1 articles
-
Differences in Clinical Outcomes between Luminal A and B Type Breast Cancers according to the St. Gallen Consensus 2013
Hyo Jung Ahn, Soo Jin Jung, Tae Hyun Kim, Min Kyung Oh, Hye-Kyoung Yoon
J Breast Cancer. 2015;18(2):149-159. doi: 10.4048/jbc.2015.18.2.149.
Reference
-
1. Zaha DC, Lazăr E, Lăzureanu C. Clinicopathologic features and five years survival analysis in molecular subtypes of breast cancer. Rom J Morphol Embryol. 2010. 51:85–89.2. Kröger N, Milde-Langosch K, Riethdorf S, Schmoor C, Schumacher M, Zander AR, et al. Prognostic and predictive effects of immunohistochemical factors in high-risk primary breast cancer patients. Clin Cancer Res. 2006. 12:159–168.
Article3. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009. 101:736–750.
Article4. Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005. 23:7350–7360.
Article5. Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Panel members. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009. 20:1319–1329.
Article6. Ahlin C, Aaltonen K, Amini RM, Nevanlinna H, Fjällskog ML, Blomqvist C. Ki67 and cyclin A as prognostic factors in early breast cancer. What are the optimal cut-off values? Histopathology. 2007. 51:491–498.
Article7. Neri A, Marrelli D, Pedrazzani C, Caruso S, De Stefano A, Mariani F, et al. Prognostic relevance of proliferative activity evaluated by Mib-1 immunostaining in node negative breast cancer. Eur J Surg Oncol. 2008. 34:1299–1303.
Article8. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. 406:747–752.
Article9. Osborne CK. Steroid hormone receptors in breast cancer management. Breast Cancer Res Treat. 1998. 51:227–238.
Article10. Hess KR, Pusztai L, Buzdar AU, Hortobagyi GN. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003. 78:105–118.
Article11. Steroid receptors in breast cancer: an NIH Consensus Development Conference, Bethesda, Maryland, June 27-29, 1979. Cancer. 1980. 46:2759–2963.12. O'Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki AB, Koziner B, et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol. 2009. 27:5208–5212.13. Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006. 24:4738–4745.
Article14. Hellemans P, van Dam PA, Weyler J, van Oosterom AT, Buytaert P, Van Marck E. Prognostic value of bcl-2 expression in invasive breast cancer. Br J Cancer. 1995. 72:354–360.
Article15. Chen HH, Su WC, Guo HR, Chang TW, Lee WY. p53 and c-erbB-2 but not bcl-2 are predictive of metastasis-free survival in breast cancer patients receiving post-mastectomy adjuvant radiotherapy in Taiwan. Jpn J Clin Oncol. 2002. 32:332–339.
Article16. Elledge RM, Green S, Howes L, Clark GM, Berardo M, Allred DC, et al. bcl-2, p53, and response to tamoxifen in estrogen receptor-positive metastatic breast cancer: a Southwest Oncology Group study. J Clin Oncol. 1997. 15:1916–1922.
Article17. Kim K, Chie EK, Han W, Noh DY, Park IA, Oh DY, et al. Prognostic value of p53 and bcl-2 expression in patients treated with breast conservative therapy. J Korean Med Sci. 2010. 25:235–239.
Article18. Ali HR, Dawson SJ, Blows FM, Provenzano E, Leung S, Nielsen T, et al. A Ki67/BCL2 index based on immunohistochemistry is highly prognostic in ER-positive breast cancer. J Pathol. 2012. 226:97–107.
Article19. Lundin J, Lundin M, Holli K, Kataja V, Elomaa L, Pylkkänen L, et al. Omission of histologic grading from clinical decision making may result in overuse of adjuvant therapies in breast cancer: results from a nationwide study. J Clin Oncol. 2001. 19:28–36.
Article20. Trudeau ME, Pritchard KI, Chapman JA, Hanna WM, Kahn HJ, Murray D, et al. Prognostic factors affecting the natural history of node-negative breast cancer. Breast Cancer Res Treat. 2005. 89:35–45.
Article21. Ivshina AV, George J, Senko O, Mow B, Putti TC, Smeds J, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006. 66:10292–10301.
Article22. Schumacher M, Schmoor C, Sauerbrei W, Schauer A, Ummenhofer L, Gatzemeier W, et al. The prognostic effect of histological tumor grade in node-negative breast cancer patients. Breast Cancer Res Treat. 1993. 25:235–245.
Article23. Roberti NE. The role of histologic grading in the prognosis of patients with carcinoma of the breast: is this a neglected opportunity? Cancer. 1997. 80:1708–1716.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The different prognostic impact of age according to individual molecular subtypes in breast cancer
- Potential Benefits of Neoadjuvant Chemotherapy in Clinically Node-Positive Luminal Subtypeâ» Breast Cancer
- Prognosis according to the timing of recurrence in breast cancer
- Prognostic Influence of BCL2 on Molecular Subtypes of Breast Cancer
- Prognostic Factors in Patients with Locally Advanced Breast Cancer Treated by Neoadjuvant Chemotherapy