J Breast Cancer.
2013 Sep;16(3):308-314. 10.4048/jbc.2013.16.3.308.
Association between BRCA Mutation Status, Pathological Findings, and Magnetic Resonance Imaging Features in Patients with Breast Cancer at Risk for the Mutation
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. doho.choi@samsung.com
- 2Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2286382
- DOI: http://doi.org/10.4048/jbc.2013.16.3.308
Abstract
- PURPOSE
We investigated the relationship between BRCA mutations, pathological findings, and magnetic resonance imaging (MRI) features in patients with breast cancer at risk for the mutation.
METHODS
Genetic testing for BRCA mutations was performed in 275 breast cancer patients with at least one risk factor for the mutation. Using the breast imaging reporting and data system MR lexicon, morphological and kinetic features were reviewed on MRI scans of 230 tumors in 209 patients. The relationship between BRCA mutations, pathologic findings, and MRI data was examined, and disease recurrence was estimated.
RESULTS
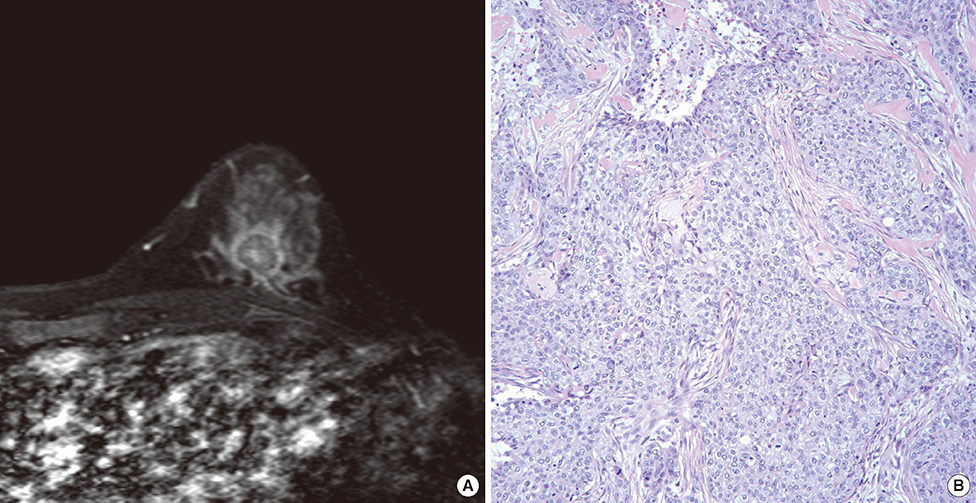
BRCA mutations were detected in 48 patients (23.0%), of which 21 (10.0%) were in BRCA1, and 25 (12.0%) in BRCA2. Additionally, two patients (1.0%) had mutations in both genes. Cancers in patients with BRCA1 mutations more frequently showed a higher nuclear grade (p=0.0041), and triple-negative (TN) phenotype (p<0.0001). On MRI scans, the cancers were seen as mass-type in 182 out of 230 lesions (79.1%), and nonmass type in 48 cases (20.9%). Among the features indentified by MRI, rim enhancement was significantly associated with molecular subtypes based on immunohistochemistry (p<0.0001), and nuclear grade (p=0.0387) in multiple logistic regression analysis. Rim enhancement on MRI, along with advanced pathologic N stage, was associated with increased disease recurrence (p=0.0023) based on multivariate analysis. However, the proportion of mass and nonmass tumors, and the distribution of morphological shape, margin, internal enhancement, and kinetic features assessed by MRI were not different according to BRCA mutation status.
CONCLUSION
BRCA1 mutations were associated with aggressive pathological characteristics, and the TN phenotype. Rim enhancement was frequently seen on MRI scans of high-grade cancers and in the TN phenotype. And it was a significant predictor of disease recurrence. However, a direct association with BRCA mutations was not observed.
MeSH Terms
Figure
Reference
-
1. Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002; 108:171–182.
Article2. Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994; 266:66–71.
Article3. Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995; 378:789–792.
Article4. Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005; 7:60.5. Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008; 26:4282–4288.
Article6. Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008; 26:2568–2581.
Article7. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007; 13(15 Pt 1):4429–4434.
Article8. Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, König R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010; 28:1450–1457.
Article9. Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004; 351:427–437.
Article10. Leach MO, Boggis CR, Dixon AK, Easton DF, Eeles RA, Evans DG, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005; 365:1769–1778.
Article11. Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009; 250:638–647.
Article12. Lee SH, Cho N, Kim SJ, Cha JH, Cho KS, Ko ES, et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol. 2008; 9:10–18.
Article13. Veltman J, Mann R, Kok T, Obdeijn IM, Hoogerbrugge N, Blickman JG, et al. Breast tumor characteristics of BRCA1 and BRCA2 gene mutation carriers on MRI. Eur Radiol. 2008; 18:931–938.
Article14. Schrading S, Kuhl CK. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008; 246:58–70.
Article15. Gilbert FJ, Warren RM, Kwan-Lim G, Thompson DJ, Eeles RA, Evans DG, et al. Cancers in BRCA1 and BRCA2 carriers and in women at high risk for breast cancer: MR imaging and mammographic features. Radiology. 2009; 252:358–368.
Article16. American College of Radiology, BI-RADS Committee. ACR BI-RADS Breast Imaging and Reporting Data System: Breast Imaging Atlas. 4th ed. Reston: American College of Radiology;2003.17. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998; 11:155–168.18. Noh JM, Choi DH, Nam SJ, Lee JE, Kim JW, Kim SW, et al. Characteristics of double heterozygosity for BRCA1 and BRCA2 germline mutations in Korean breast cancer patients. Breast Cancer Res Treat. 2012; 131:217–222.
Article19. Son BH, Ahn SH, Kim SW, Kang E, Park SK, Lee MH, et al. Prevalence of BRCA1 and BRCA2 mutations in non-familial breast cancer patients with high risks in Korea: the Korean Hereditary Breast Cancer (KOHBRA) Study. Breast Cancer Res Treat. 2012; 133:1143–1152.
Article20. Han SA, Kim SW, Kang E, Park SK, Ahn SH, Lee MH, et al. The prevalence of BRCA mutations among familial breast cancer patients in Korea: results of the Korean Hereditary Breast Cancer study. Fam Cancer. 2013; 12:75–81.
Article21. Sickles EA. Nonpalpable, circumscribed, noncalcified solid breast masses: likelihood of malignancy based on lesion size and age of patient. Radiology. 1994; 192:439–442.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Magnetic Resonance Imaging of Breast Cancer Patients with BRCA Mutation
- The Incidence of Occult Malignancy in Contralateral Risk Reducing Mastectomy Among Affected Breast Cancer Gene Mutation Carriers in South Korea
- Experience with Bilateral Risk-Reducing Mastectomy for an Unaffected BRCA Mutation Carrier
- Occurrence of contralateral breast cancer in a BRCA-positive breast cancer patient who underwent free TRAM flap reconstruction: a case report
- Is the BRCA Germline Mutation a Prognostic Factor in Korean Patients with Early-onset Breast Carcinomas?