Infect Chemother.
2013 Jun;45(2):117-136. 10.3947/ic.2013.45.2.117.
Genomic Basis for Methicillin Resistance in Staphylococcus aureus
- Affiliations
-
- 1Department of Bacteriology, Juntendo University, Tokyo, Japan. khiram06@juntendo.ac.jp
- 2Research Center for Infection Control Science, Juntendo University, Tokyo, Japan.
- 3Department of Veterinary Science, Rakuno Gakuen University, Hokkaido, Japan.
- 4National Institute of Infectious Diseases, Tokyo, Japan.
- KMID: 2284994
- DOI: http://doi.org/10.3947/ic.2013.45.2.117
Abstract
- Since the discovery of the first strain in 1961 in England, MRSA, the most notorious multidrug-resistant hospital pathogen, has spread all over the world. MRSA repeatedly turned down the challenges by number of chemotherapeutics, the fruits of modern organic chemistry. Now, we are in short of effective therapeutic agents against MRSA prevailing among immuno-compromised patients in the hospital. On top of this, we recently became aware of the rise of diverse clones of MRSA, some of which have increased pathogenic potential compared to the classical hospital-associated MRSA, and the others from veterinary sources. They increased rapidly in the community, and started menacing otherwise healthy individuals by causing unexpected acute infection. This review is intended to provide a whole picture of MRSA based on its genetic makeup as a versatile pathogen and our tenacious colonizer.
Keyword
MeSH Terms
Figure
Reference
-
1. Hiramatsu K. Molecular evolution of MRSA. Microbiol Immunol. 1995; 39:531–543. PMID: 7494490.
Article2. Jevons MP. 'Celbenin'-resistant staphylococci. BMJ. 1961; 1:124–125.3. Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci U S A. 2002; 99:7687–7692. PMID: 12032344.4. Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997; 40:135–136. PMID: 9249217.5. Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK. Vancomycin-Resistant Staphylococcus aureus Investigative Team. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003; 348:1342–1347. PMID: 12672861.6. Udo EE, Pearman JW, Grubb WB. Genetic analysis of community isolates of methicillin-resistant Staphylococcus aureus in Western Australia. J Hosp Infect. 1993; 25:97–108. PMID: 7903093.7. Riley TV, Pearman JW, Rouse IL. Changing epidemiology of methicillin-resistant Staphylococcus aureus in Western Australia. Med J Aust. 1995; 163:412–414. PMID: 7476610.8. Moreno F, Crisp C, Jorgensen JH, Patterson JE. Methicillin-resistant Staphylococcus aureus as a community organism. Clin Infect Dis. 1995; 21:1308–1312. PMID: 8589164.9. Aubry-Damon H, Legrand P, Brun-Buisson C, Astier A, Soussy CJ, Leclercq R. Reemergence of gentamicin-susceptible strains of methicillin-resistant Staphylococcus aureus: roles of an infection control program and changes in aminoglycoside use. Clin Infect Dis. 1997; 25:647–653. PMID: 9314454.10. Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002; 359:1819–1827. PMID: 12044378.
Article11. Takeuchi F, Watanabe S, Baba T, Yuzawa H, Ito T, Morimoto Y, Kuroda M, Cui L, Takahashi M, Ankai A, Baba S, Fukui S, Lee JC, Hiramatsu K. Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol. 2005; 187:7292–7308. PMID: 16237012.12. Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol Microbiol. 1998; 29:527–543. PMID: 9720870.13. Novick RP, Subedi A. The SaPIs: mobile pathogenicity islands of Staphylococcus. Chem Immunol Allergy. 2007; 93:42–57. PMID: 17369699.14. O'Neill AJ, Larsen AR, Skov R, Henriksen AS, Chopra I. Characterization of the epidemic European fusidic acid-resistant impetigo clone of Staphylococcus aureus. J Clin Microbiol. 2007; 45:1505–1510. PMID: 17344365.15. Ubeda C, Maiques E, Tormo MA, Campoy S, Lasa I, Barbé J, Novick RP, Penadés JR. SaPI operon I is required for SaPI packaging and is controlled by LexA. Mol Microbiol. 2007; 65:41–50. PMID: 17581119.16. Tormo MA, Ferrer MD, Maiques E, Ubeda C, Selva L, Lasa I, Calvete JJ, Novick RP, Penadés JR. Staphylococcus aureus pathogenicity island DNA is packaged in particles composed of phage proteins. J Bacteriol. 2008; 190:2434–2440. PMID: 18223072.17. Chen J, Novick RP. Phage-mediated intergeneric transfer of toxin genes. Science. 2009; 323:139–141. PMID: 19119236.
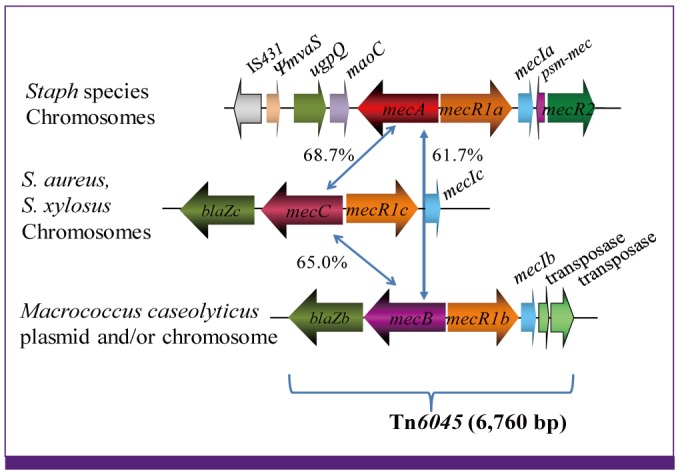
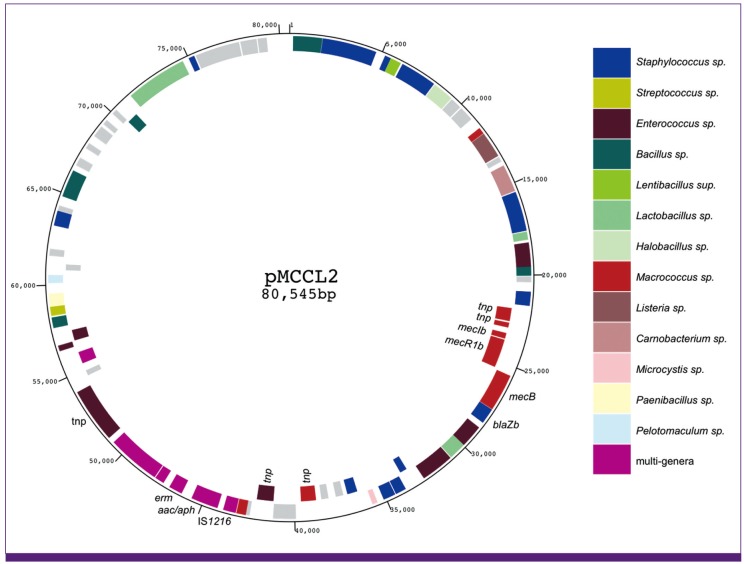
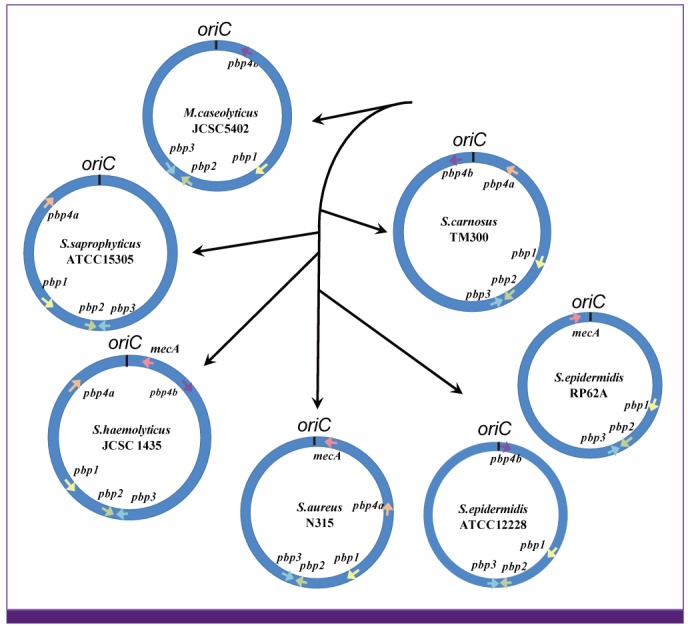
Article18. Baba T, Kuwahara-Arai K, Uchiyama I, Takeuchi F, Ito T, Hiramatsu K. Complete genome sequence determination of a Macrococcus caseolyticus strain JSCS5402 reflecting the ancestral genome of the human pathogenic staphylococci. J Bacteriol. 2009; 191:1180–1190. PMID: 19074389.19. Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005; 187:2426–2438. PMID: 15774886.20. Lina G, Bohach GA, Nair SP, Hiramatsu K, Jouvin-Marche E, Mariuzza R. International Nomenclature Committee for Staphylococcal Superantigens. Standard nomenclature for the superantigens expressed by Staphylococcus. J Infect Dis. 2004; 189:2334–2336. PMID: 15181583.21. Langley R, Wines B, Willoughby N, Basu I, Proft T, Fraser JD. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc alpha RI binding and serum killing of bacteria. J Immunol. 2005; 174:2926–2933. PMID: 15728504.22. Baba T, Takeuchi F, Kuroda M, Ito T, Yuzawa H, Hiramatsu K. The Staphylococcus aureus genome. In : Ala'Aldeen D, Hiramatsu K, editors. Staphylococcus aureus Molecular and Clinical Aspects. Chichester, UK: Horwood Publishing;2004. p. 66–153.23. Yamaguchi T, Nishifuji K, Sasaki M, Fudaba Y, Aepfelbacher M, Takata T, Ohara M, Komatsuzawa H, Amagai M, Sugai M. Identification of the Staphylococcus aureus etd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect Immun. 2002; 70:5835–5845. PMID: 12228315.24. Manna AC, Cheung AL. sarU, a sarA homolog, is repressed by SarT and regulates virulence genes in Staphylococcus aureus. Infect Immun. 2003; 71:343–353. PMID: 12496184.25. Jamaluddin TZ, Kuwahara-Arai K, Hisata K, Terasawa M, Cui L, Baba T, Sotozono C, Kinoshita S, Ito T, Hiramatsu K. Extreme genetic diversity of methicillin-resistant Staphylococcus epidermidis strains disseminated among healthy Japanese children. J Clin Microbiol. 2008; 46:3778–3783. PMID: 18832123.26. Watanabe S, Ito T, Morimoto Y, Takeuchi F, Hiramatsu K. Precise excision and self-integration of a composite transposon as a model for spontaneous large-scale chromosome inversion/deletion of the Staphylococcus haemolyticus clinical strain JCSC1435. J Bacteriol. 2007; 189:2921–2925. PMID: 17237177.27. Gustafson J, Strässle A, Hächler H, Kayser FH, Berger-Bächi B. The femC locus of Staphylococcus aureus required for methicillin resistance includes the glutamine synthetase operon. J Bacteriol. 1994; 176:1460–1467. PMID: 7509336.28. Maki H, Murakami K. Formation of potent hybrid promoters of the mutant llm gene by IS256 transposition in methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997; 179:6944–6948. PMID: 9371438.29. Hiramatsu K. Vancomycin resistance in staphylococci. Drug Resist Updat. 1998; 1:135–150. PMID: 16904400.
Article30. Kozitskaya S, Cho SH, Dietrich K, Marre R, Naber K, Ziebuhr W. The bacterial insertion sequence element IS256 occurs preferentially in nosocomial Staphylococcus epidermidis isolates: association with biofilm formation and resistance to aminoglycosides. Infect Immun. 2004; 72:1210–1215. PMID: 14742578.31. Rosenstein R, Nerz C, Biswas L, Resch A, Raddatz G, Schuster SC, Gätz F. Genome analysis of the meat starter culture bacterium Staphylococcus carnosus TM300. Appl Environ Microbiol. 2009; 75:811–822. PMID: 19060169.32. Ito T, Katayama Y, Hiramatsu K. Cloning and nucleotide sequence determination of the entire mec DNA of pre-methicillin-resistant Staphylococcus aureus N315. Antimicrob Agents Chemother. 1999; 43:1449–1458. PMID: 10348769.33. Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2000; 44:1549–1555. PMID: 10817707.34. Sjöström JE, Löfdahl S, Philipson L. Transformation reveals a chromosomal locus of the gene(s) for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1975; 123:905–915. PMID: 125746.35. Kuhl SA, Pattee PA, Baldwin JN. Chromosomal map location of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1978; 135:460–465. PMID: 249312.36. Stewart GC, Rosenblum ED. Genetic behavior of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1980; 144:1200–1202. PMID: 6254946.37. Brown DF, Reynolds PE. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980; 122:275–278. PMID: 7202719.38. Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984; 158:513–516. PMID: 6563036.39. Utsui Y, Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985; 28:397–403. PMID: 3878127.40. Ubukata K, Yamashita N, Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985; 27:851–857. PMID: 3848294.
Article41. Matsuhashi M, Song MD, Ishino F, Wachi M, Doi M, Inoue M, Ubukata K, Yamashita N, Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986; 167:975–980. PMID: 3638304.42. Song MD, Wachi M, Doi M, Ishino F, Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987; 221:167–171. PMID: 3305073.43. Tesch W, Ryffel C, Strässle A, Kayser FH, Berger-Bächi B. Evidence of a novel staphylococcal mec-encoded element (mecR) controlling expression of penicillin-binding protein 2'. Antimicrob Agents Chemother. 1990; 34:1703–1706. PMID: 2285282.44. Hürlimann-Dalel RL, Ryffel C, Kayser FH, Berger-Bächi B. Survey of the methicillin resistance-associated genes mecA, mecR1-mecI, and femA-femB in clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1992; 36:2617–2621. PMID: 1362343.45. Suzuki E, Kuwahara-Arai K, Richardson JF, Hiramatsu K. Distribution of mec regulator genes in methicillin-resistant Staphylococcus clinical strains. Antimicrob Agents Chemother. 1993; 37:1219–1226. PMID: 8328773.46. Ryffel C, Kayser FH, Berger-Bächi B. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrob Agents Chemother. 1992; 36:25–31. PMID: 1375449.47. Hiramatsu K, Asada K, Suzuki E, Okonogi K, Yokota T. Molecular cloning and nucleotide sequence determination of the regulator region of mecA gene in methicillin-resistant Staphylococcus aureus (MRSA). FEBS Lett. 1992; 298:133–136. PMID: 1544435.48. Kuwahara-Arai K, Kondo N, Hori S, Tateda-Suzuki E, Hiramatsu K. Suppression of methicillin resistance in a mecA-containing pre-methicillin-resistant Staphylococcus aureus strain is caused by the mecI-mediated repression of PBP 2' production. Antimicrob Agents Chemother. 1996; 40:2680–2685. PMID: 9124822.49. Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, Hiramatsu K. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001; 45:1323–1336. PMID: 11302791.50. Hiramatsu K, Kondo N, Ito T. Genetic basis for molecular epidemiology of MRSA. J Infect Chemother. 1996; 2:117–129.
Article51. Stokes HW, Hall RM. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989; 3:1669–1683. PMID: 2560119.
Article52. Hiramatsu K, Katayama Y, Yuzawa H, Ito T. Molecular genetics of methicillin-resistant Staphylococcus aureus. Int J Med Microbiol. 2002; 292:67–74. PMID: 12195737.53. Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, Jamklang M, Chavalit T, Song JH, Hiramatsu K. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal for a new nomenclature for SCCmec elements. Antimicrob Agents Chemother. 2006; 50:1001–1012. PMID: 16495263.54. Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, Hiramatsu K. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother. 2007; 51:264–274. PMID: 17043114.55. Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, Daum RS, Hiramatsu K. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002; 46:1147–1152. PMID: 11897611.56. Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004; 48:2637–2651. PMID: 15215121.57. International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009; 53:4961–4967. PMID: 19721075.58. Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O'Brien FG, Coombs GW, Pearman JW, Tenover FC, Kapi M, Tiensasitorn C, Ito T, Hiramatsu K. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol. 2002; 40:4289–4294. PMID: 12409412.59. Hisata K, Kuwahara-Arai K, Yamanoto M, Ito T, Nakatomi Y, Cui L, Baba T, Terasawa M, Sotozono C, Kinoshita S, Yamashiro Y, Hiramatsu K. Dissemination of methicillin-resistant staphylococci among healthy Japanese children. J Clin Microbiol. 2005; 43:3364–3372. PMID: 16000461.
Article60. Ruppé E, Barbier F, Mesli Y, Maiga A, Cojocaru R, Benkhalfat M, Benchouk S, Hassaine H, Maiga I, Diallo A, Koumaré AK, Ouattara K, Soumaré S, Dufourcq JB, Nareth C, Sarthou JL, Andremont A, Ruimy R. Diversity of staphylococcal cassette chromosome mec structures in methicillin-resistant Staphylococcus epidermidis and Staphylococcus haemolyticus strains among outpatients from four countries. Antimicrob Agents Chemother. 2009; 53:442–449. PMID: 19001111.61. Hiramatsu K, Cui L, Kuroda M, Ito T. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2001; 9:486–493. PMID: 11597450.62. Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001; 7:178–182. PMID: 11294701.63. Zhang HZ, Hackbarth CJ, Chansky KM, Chambers HF. A proteolytic transmembrane signaling pathway and resistance to beta-lactams in staphylococci. Science. 2001; 291:1962–1965. PMID: 11239156.64. Hiramatsu K, Suzuki E, Takayama H, Katayama Y, Yokota T. Role of penicillinase plasmids in the stability of the mecA gene in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990; 34:600–604. PMID: 2344167.65. Wada A, Katayama Y, Hiramatsu K, Yokota T. Southern hybridization analysis of the mecA deletion from methicillin-resistant Staphylococcus aureus. Biochem Biophys Res Commun. 1991; 176:1319–1325. PMID: 2039513.66. Lee SM, Ender M, Adhikari R, Smith JM, Berger-Bächi B, Cook GM. Fitness cost of staphylococcal cassette chromosome mec in methicillin-resistant Staphylococcus aureus by way of continuous culture. Antimicrob Agents Chemother. 2007; 51:1497–1499. PMID: 17283194.67. Chatterjee SS, Chen L, Joo HS, Cheung GY, Kreiswirth BN, Otto M. Distribution and regulation of the mobile genetic element-encoded phenol-soluble modulin PSM-mec in methicillin-resistant Staphylococcus aureus. PLoS One. 2011; 6:e28781. PMID: 22174895.68. Kaito C, Sekimizu K. Colony spreading in Staphylococcus aureus. J Bacteriol. 2007; 189:2553–2557. PMID: 17194792.69. Kaito C, Omae Y, Matsumoto Y, Nagata M, Yamaguchi H, Aoto T, Ito T, Hiramatsu K, Sekimizu K. A novel gene, fudoh, in the SCCmec region suppresses the colony spreading ability and virulence of Staphylococcus aureus. PLoS ONE. 2008; 3:e3921. PMID: 19079549.70. Hongo I, Baba T, Oishi K, Morimoto Y, Ito T, Hiramatsu K. Phenol-soluble modulin alpha 3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J Infect Dis. 2009; 200:715–723. PMID: 19653829.71. Kaito C, Saito Y, Nagano G, Ikuo M, Omae Y, Hanada Y, Han X, Kuwahara-Arai K, Hishinuma T, Baba T, Ito T, Hiramatsu K, Sekimizu K. Transcription and translation products of the cytolysin gene psm-mec on the mobile genetic element SCCmec regulate Staphylococcus aureus virulence. PLoS Pathog. 2011; 7:e1001267. PMID: 21304931.72. Kaito C, Saito Y, Ikuo M, Omae Y, Mao H, Nagano G, Fujiyuki T, Numata S, Han X, Obata K, Hasegawa S, Yamaguchi H, Inokuchi K, Ito T, Hiramatsu K, Sekimizu K. Mobile genetic element SCCmec-encoded psm-mec RNA suppresses translation of agrA and attenuates MRSA virulence. PLoS Pathog. 2013; 9:e1003269. PMID: 23592990.73. Luong TT, Ouyang S, Bush K, Lee CY. Type 1 capsule genes of Staphylococcus aureus are carried in a staphylococcal cassette chromosome genetic element. J Bacteriol. 2002; 184:3623–3629. PMID: 12057957.74. Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, Barron A, Bason N, Bentley SD, Chillingworth C, Chillingworth T, Churcher C, Clark L, Corton C, Cronin A, Doggett J, Dowd L, Feltwell T, Hance Z, Harris B, Hauser H, Holroyd S, Jagels K, James KD, Lennard N, Line A, Mayes R, Moule S, Mungall K, Ormond D, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Sharp S, Simmonds M, Stevens K, Whitehead S, Barrell BG, Spratt BG, Parkhill J. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A. 2004; 101:9786–9791. PMID: 15213324.75. Zhang YQ, Ren SX, Li HL, Wang YX, Fu G, Yang J, Qin ZQ, Miao YG, Wang WY, Chen RS, Shen Y, Chen Z, Yuan ZH, Zhao GP, Qu D, Danchin A, Wen YM. Genome-based analysis of virulence genes in a non-biofilm-forming Staphylococcus epidermidis strain (ATCC 12228). Mol Microbiol. 2003; 49:1577–1593. PMID: 12950922.76. Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, Lin F, Lin J, Carleton HA, Mongodin EF, Sensabaugh GF, Perdreau-Remington F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006; 367:731–739. PMID: 16517273.77. Wu S, Piscitelli C, de Lencastre H, Tomasz A. Tracking the evolutionary origin of the methicillin resistance gene: cloning and sequencing of a homologue of mecA from a methicillin susceptible strain of Staphylococcus sciuri. Microb Drug Resist. 1996; 2:435–441. PMID: 9158816.78. Tsubakishita S, Kuwahara-Arai K, Sasaki T, Hiramatsu K. Origin and molecular evolution of the determinant of methicillin resistance in staphylococci. Antimicrob Agents Chemother. 2010; 54:4352–4359. PMID: 20679504.
Article79. Hiramatsu K, Tsubakishita S, Matsuo M, Sasaki T. Molecular Evolution of MRSA 2010. In : 12th Western Pacific Congress on Chemotherapy and Infectious Diseases; 2011 Dec 2-5; Singapore. p. 11–18.80. Tsubakishita S, Kuwahara-Arai K, Baba T, Hiramatsu K. Staphylococcal cassette chromosome mec-like element in Macrococcus caseolyticus. Antimicrob Agents Chemother. 2010; 54:1469–1475. PMID: 20086147.81. García-Álvarez L, Holden MT, Lindsay H, Webb CR, Brown DF, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RL, Larsen AR, Skov RL, Peacock SJ, Maskell DJ, Holmes MA. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis. 2011; 11:595–603. PMID: 21641281.82. Shore AC, Deasy EC, Slickers P, Brennan G, O'Connell B, Monecke S, Ehricht R, Coleman DC. Detection of staphylococcal cassette chromosome mec type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011; 55:3765–3773. PMID: 21636525.83. Harrison EM, Paterson GK, Holden MT, Morgan FJ, Larsen AR, Petersen A, Leroy S, De Vliegher S, Perreten V, Fox LK, Lam TJ, Sampimon OC, Zadoks RN, Peacock SJ, Parkhill J, Holmes MA. A Staphylococcus xylosus isolate with a new mecC allotype. Antimicrob Agents Chemother. 2013; 57:1524–1528. PMID: 23274660.84. Schnellmann C, Gerber V, Rossano A, Jaquier V, Panchaud Y, Doherr MG, Thomann A, Straub R, Perreten V. Presence of new mecA and mph(C) variants conferring antibiotic resistance in Staphylococcus spp. isolated from the skin of horses before and after clinic admission. J Clin Microbiol. 2006; 44:4444–4454. PMID: 17005735.85. Fontana R, Ligozzi M, Pittaluga F, Satta G. Intrinsic penicillin resistance in enterococci. Microb Drug Resist. 1996; 2:209–213. PMID: 9158761.
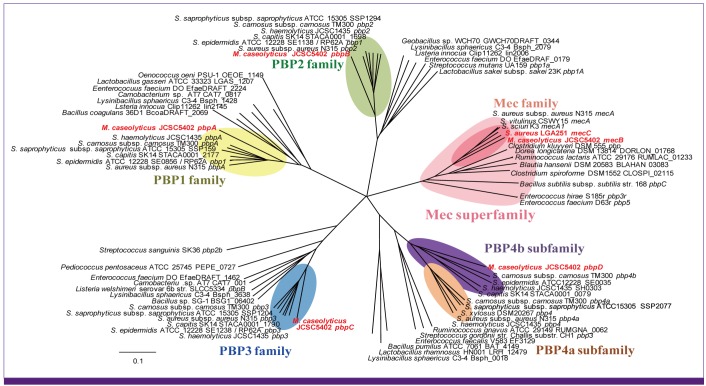
Article86. Williamson R, le Bouguénec C, Gutmann L, Horaud T. One or two low affinity penicillin-binding proteins may be responsible for the range of susceptibility of Enterococcus faecium to benzylpenicillin. J Gen Microbiol. 1985; 131:1933–1940. PMID: 3850924.87. el Kharroubi A, Jacques P, Piras G, Van Beeumen J, Coyette J, Ghuysen JM. The Enterococcus hirae R40 penicillin-binding protein 5 and the methicillin-resistant Staphylococcus aureus penicillin-binding protein 2' are similar. Biochem J. 1991; 280:463–469. PMID: 1747121.88. Piras G, Raze D, el Kharroubi A, Hastir D, Englebert S, Coyette J, Ghuysen JM. Cloning and sequencing of the low-affinity penicillin-binding protein 3r-encoding gene of Enterococcus hirae S185: modular design and structural organization of the protein. J Bacteriol. 1993; 175:2844–2852. PMID: 8491705.89. Mongkolrattanothai K, Boyle S, Murphy TV, Daum RS. Novel non-mecA-containing staphylococcal chromosomal cassette composite island containing pbp4 and tagF genes in a commensal staphylococcal species: a possible reservoir for antibiotic resistance islands in Staphylococcus aureus. Antimicrob Agents Chemother. 2004; 48:1823–1836. PMID: 15105141.90. Wyke AW, Ward JB, Hayes MV, Curtis NA. A role in vivo for penicillin-binding protein-4 of Staphylococcus aureus. Eur J Biochem. 1981; 119:389–393. PMID: 7308191.91. Henze UU, Roos M, Berger-Bächi B. Effects of penicillin-binding protein 4 overproduction in Staphylococcus aureus. Microb Drug Resist. 1996; 2:193–199. PMID: 9158759.92. Yamakawa J, Aminaka M, Okuzumi K, Kobayashi H, Katayama Y, Kondo S, Nakamura A, Oguri T, Hori S, Cui L, Ito T, Jin J, Kurosawa H, Kaneko K, Hiramatsu K. Heterogeneously vancomycin-intermediate Staphylococcus aureus (hVISA) emerged before the clinical introduction of vancomycin in Japan: a retrospective study. J Infect Chemother. 2012; 18:406–409. PMID: 22033576.93. Ryffel C, Strässle A, Kayser FH, Berger-Bächi B. Mechanisms of heteroresistance in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1994; 38:724–728. PMID: 8031036.94. Kuroda M, Kuroda H, Oshima T, Takeuchi F, Mori H, Hiramatsu K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol. 2003; 49:807–821. PMID: 12864861.95. Kuroda M, Kuwahara-Arai K, Hiramatsu K. Identification of the up- and down-regulated genes in vancomycin-resistant Staphylococcus aureus strains Mu3 and Mu50 by cDNA differential hybridization method. Biochem Biophys Res Commun. 2000; 269:485–490. PMID: 10708580.96. Aiba Y, Katayama Y, Hishinuma T, Murakami-Kuroda H, Cui L, Hiramatsu K. Mutation of RNA polymerase β-subunit gene promotes hetero-to-homo conversion of β-lactam resistance of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. [Submitted].97. Kondo N, Kuwahara-Arai K, Kuroda-Murakami H, Tateda-Suzuki E, Hiramatsu K. Eagle-type methicillin resistance: new phenotype of high methicillin resistance under mec regulator gene control. Antimicrob Agents Chemother. 2001; 45:815–824. PMID: 11181367.98. Matsuo M, Hishinuma T, Katayama Y, Cui L, Kapi M, Hiramatsu K. Mutation of RNA polymerase beta subunit (rpoB) promotes hVISA-to-VISA phenotypic conversion of strain Mu3. Antimicrob Agents Chemother. 2011; 55:4188–4195. PMID: 21746940.99. Hiramatsu K, Igarashi M, Morimoto Y, Baba T, Umekita M, Akamatsu Y. Curing bacteria of antibiotic resistance: reverse antibiotics, a novel class of antibiotics in nature. Int J Antimicrob Agents. 2012; 39:478–485. PMID: 22534508.
Article100. Uchiyama I. MBGD: a platform for microbial comparative genomics based on the automated construction of orthologous groups. Nucleic Acids Res. 2007; 35:D343–D346. PMID: 17135196.
Article101. Uchiyama I. Hierarchical clustering algorithm for comprehensive orthologous-domain classification in multiple genomes. Nucleic Acids Res. 2006; 34:647–658. PMID: 16436801.
Article102. Clarke SR, Harris LG, Richards RG, Foster SJ. Analysis of Ebh, a 1.1-megadalton cell wall-associated fibronectin-binding protein of Staphylococcus aureus. Infect Immun. 2002; 70:6680–6687. PMID: 12438342.103. Herron-Olson L, Fitzgerald JR, Musser JM, Kapur V. Molecular correlates of host specialization in Staphylococcus aureus. PLoS One. 2007; 2:e1120. PMID: 17971880.104. Uchiyama I. Multiple genome alignment for identifying the core structure among moderately related microbial genomes. BMC Genomics. 2008; 9:515. PMID: 18976470.
Article105. Yarwood JM, McCormick JK, Paustian ML, Orwin PM, Kapur V, Schlievert PM. Characterization and expression analysis of Staphylococcus aureus pathogenicity island 3. Implications for the evolution of staphylococcal pathogenicity islands. J Biol Chem. 2002; 277:13138–13147. PMID: 11821418.106. Highlander SK, Hultén KG, Qin X, Jiang H, Yerrapragada S, Mason EO Jr, Shang Y, Williams TM, Fortunov RM, Liu Y, Igboeli O, Petrosino J, Tirumalai M, Uzman A, Fox GE, Cardenas AM, Muzny DM, Hemphill L, Ding Y, Dugan S, Blyth PR, Buhay CJ, Dinh HH, Hawes AC, Holder M, Kovar CL, Lee SL, Liu W, Nazareth LV, Wang Q, Zhou J, Kaplan SL, Weinstock GM. Subtle genetic changes enhance virulence of methicillin resistant and sensitive Staphylococcus aureus. BMC Microbiol. 2007; 7:99. PMID: 17986343.
Article107. Fitzgerald JR, Monday SR, Foster TJ, Bohach GA, Hartigan PJ, Meaney WJ, Smyth CJ. Characterization of a putative pathogenicity island from bovine Staphylococcus aureus encoding multiple superantigens. J Bacteriol. 2001; 183:63–70. PMID: 11114901.108. Ubeda C, Tormo MA, Cucarella C, Trotonda P, Foster TJ, Lasa I, Penadés JR. Sip, an integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Mol Microbiol. 2003; 49:193–210. PMID: 12823821.109. Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, Cui L, Oguchi A, Aoki K, Nagai Y, Lian J, Ito T, Kanamori M, Matsumaru H, Maruyama A, Murakami H, Hosoyama A, Mizutani-Ui Y, Takahashi NK, Sawano T, Inoue R, Kaito C, Sekimizu K, Hirakawa H, Kuhara S, Goto S, Yabuzaki J, Kanehisa M, Yamashita A, Oshima K, Furuya K, Yoshino C, Shiba T, Hattori M, Ogasawara N, Hayashi H, Hiramatsu K. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet. 2001; 357:1225–1240. PMID: 11418146.110. Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. J Bacteriol. 2008; 190:300–310. PMID: 17951380.111. Subedi A, Ubeda C, Adhikari RP, Penadés JR, Novick RP. Sequence analysis reveals genetic exchanges and intraspecific spread of SaPI2, a pathogenicity island involved in menstrual toxic shock. Microbiology. 2007; 153:3235–3245. PMID: 17906123.
Article112. Kwan T, Liu J, DuBow M, Gros P, Pelletier J. The complete genomes and proteomes of 27 Staphylococcus aureus bacteriophages. Proc Natl Acad Sci U S A. 2005; 102:5174–5179. PMID: 15788529.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Multidrug Resistant Patterns and Associated - genes of Methicillin Resistant Staphylococcus aureus ( MRSA ) Isolated from Clinical Specimens
- A statistical analysis of methicillin-resistant staphylococcus aureus
- A case of multiple furunculosis caused by methicillin-resistant staphylococcs aureus
- Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)
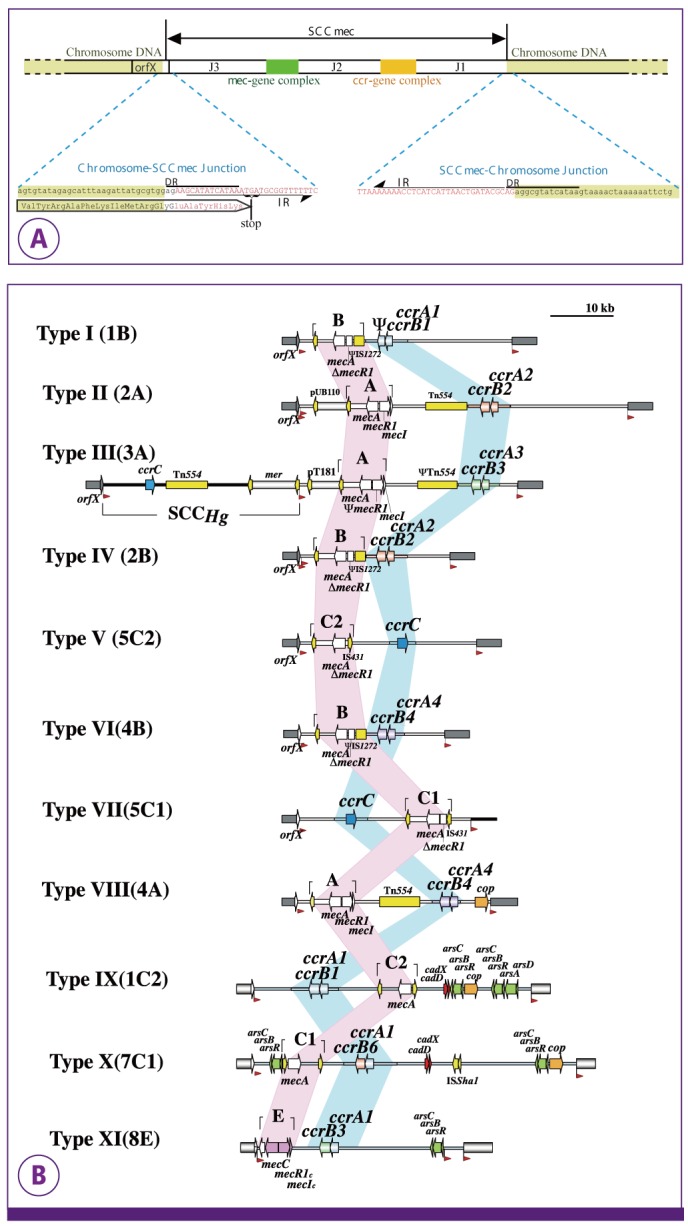
- A Survey for Methicillin - Resistant Staphylococcus Aureus