Ewha Med J.
2013 Mar;36(1):18-25. 10.12771/emj.2013.36.1.18.
Clinicopathologic Charateristics and Gallbladder Dysfunction in Patients with Endoscopic Bile Reflux
- Affiliations
-
- 1Department of Internal Medicine, Ewha Womans University School of Medicine, Seoul, Korea. syy@ewha.ac.kr
- KMID: 2284011
- DOI: http://doi.org/10.12771/emj.2013.36.1.18
Abstract
OBJECTIVES
To investigate clinicopathologic findings and gallbladder (GB) function in patients with endoscopic bile reflux at outpatients clinic.
METHODS
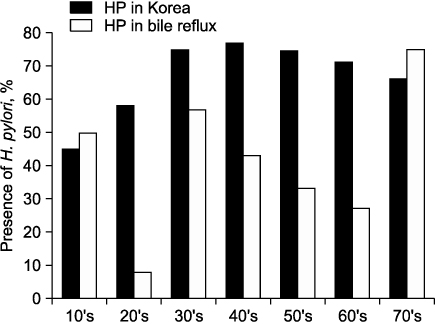
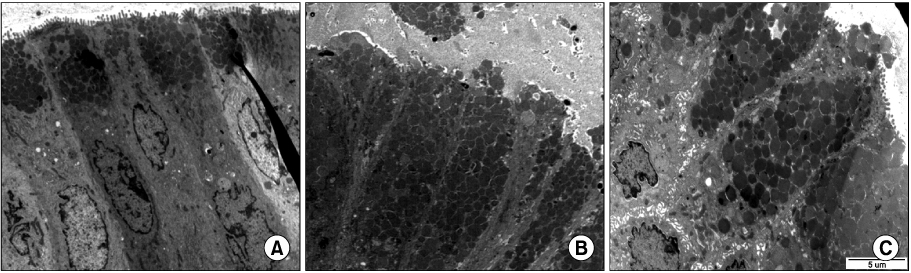
We classified endoscopic bile reflux into two groups by bile reflux index (BRI). Those who scored above 14 were the BRI (+) group, and those below 14 were the BRI (-) group. We analyzed clinical characteristics, endoscopic findings including Helicobacter pylori, GB function by DISIDA scan, and electron microscope (EM) findings of endoscopic bile reflux. And we compared clinicopathologic characteristics and GB function between two groups.
RESULTS
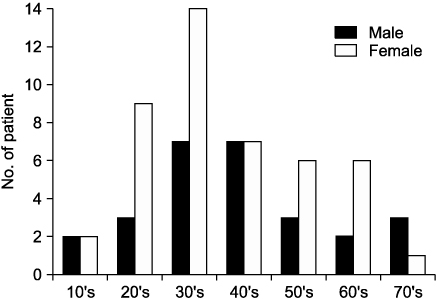
Endoscopic bile reflux identified in 9.7% of all cases with gastrointestinal symptoms. There are cholecystectomy in 6.7%, gastrectomy in 2.7%, and GB dysfunction in 20.0%. They had prominent gastrointestinal symptoms with variable endoscopic findings. Foveolar hyperplasia is the most common pathologic finding and H. pylori colonization of the stomach was inhibited in cases of bile reflux gastritis. Bile reflux also had distinguishable ultra-structural changes identifiable by EM. BRI (+) group had more old age, GB dysfunction than BRI (-) group. Clinical symptoms and endoscopic findings did not differ between the two groups of endoscopic bile reflux.
CONCLUSION
Endoscopic bile reflux was common findings with young adults (30's) at outpatients clinic. Foveolar hyperplasia is common pathologic finding. GB dysfunction were identified as significant risk factors for BRI (+) group.
Keyword
MeSH Terms
Figure
Reference
-
1. Coleman R, Iqbal S, Godfrey PP, Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979. 178:201–208.2. Moschetta A, vanBerge-Henegouwen GP, Portincasa P, Renooij WL, Groen AK, van Erpecum KJ. Hydrophilic bile salts enhance differential distribution of sphingomyelin and phosphatidylcholine between micellar and vesicular phases: potential implications for their effects in vivo. J Hepatol. 2001. 34:492–499.3. Moschetta A, Frederik PM, Portincasa P, vanBerge-Henegouwen GP, van Erpecum KJ. Incorporation of cholesterol in sphingomyelin-phosphatidylcholine vesicles has profound effects on detergent-induced phase transitions. J Lipid Res. 2002. 43:1046–1053.4. Velardi AL, Groen AK, Elferink RP, van der Meer R, Palasciano G, Tytgat GN. Cell type-dependent effect of phospholipid and cholesterol on bile salt cytotoxicity. Gastroenterology. 1991. 101:457–464.5. Moschetta A, vanBerge-Henegouwen GP, Portincasa P, Palasciano G, Groen AK, van Erpecum KJ. Sphingomyelin exhibits greatly enhanced protection compared with egg yolk phosphatidylcholine against detergent bile salts. J Lipid Res. 2000. 41:916–924.6. Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009. 15:3329–3340.7. Hoare AM, Jones EL, Alexander-Williams J, Hawkins CF. Symptomatic significance of gastric mucosal changes after surgery for peptic ulcer. Gut. 1977. 18:295–300.8. Fountos A, Chrysos E, Tsiaoussis J, Karkavitsas N, Zoras OJ, Katsamouris A, et al. Duodenogastric reflux after biliary surgery: scintigraphic quantification and improvement with erythromycin. ANZ J Surg. 2003. 73:400–403.9. Abu Farsakh NA, Stietieh M, Abu Farsakh FA. The postcholecystectomy syndrome: a role for duodenogastric reflux. J Clin Gastroenterol. 1996. 22:197–201.10. Vere CC, Cazacu S, Comănescu V, Mogoantă L, Rogoveanu I, Ciurea T. Endoscopical and histological features in bile reflux gastritis. Rom J Morphol Embryol. 2005. 46:269–274.11. Taşkin V, Sedele M, Saka O, Kantarçeken B. The effect of duodenogastric reflux on Helicobacter pylori presence and gastric histopathologic changes. Turk J Gastroenterol. 2003. 14:239–242.12. Lujan-Mompean JA, Robles-Campos R, Parrilla-Paricio P, Liron-Ruiz R, Torralba-Martinez JA, Cifuentes-Tebar J. Duodenogastric reflux in patients with biliary lithiasis before and after cholecystectomy. Surg Gynecol Obstet. 1993. 176:116–118.13. Bechi P, Cianchi F. Technical aspects and clinical indications of 24-hour intragastric bile monitoring. Hepatogastroenterology. 1999. 46:54–59.14. Dixon MF, Mapstone NP, Neville PM, Moayyedi P, Axon AT. Bile reflux gastritis and intestinal metaplasia at the cardia. Gut. 2002. 51:351–355.15. Sobala GM, King RF, Axon AT, Dixon MF. Reflux gastritis in the intact stomach. J Clin Pathol. 1990. 43:303–306.16. Kakhki VR, Zakavi SR, Davoudi Y. Normal values of gallbladder ejection fraction using 99mTc-sestamibi scintigraphy after a fatty meal formula. J Gastrointestin Liver Dis. 2007. 16:157–161.17. Sobala GM, O'Connor HJ, Dewar EP, King RF, Axon AT, Dixon MF. Bile reflux and intestinal metaplasia in gastric mucosa. J Clin Pathol. 1993. 46:235–240.18. Kim JH, Kim HY, Kim NY, Kim SW, Kim JG, Kim JJ, et al. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001. 16:969–975.19. Koek GH, Vos R, Sifrim D, Cuomo R, Janssens J, Tack J. Mechanisms underlying duodeno-gastric reflux in man. Neurogastroenterol Motil. 2005. 17:191–199.20. Shih WJ, Coupal JJ, Domstad PA, Ram MD, DeLand FH. Disorders of gallbladder function related to duodenogastric reflux in technetium-99m DISIDA hepatobiliary scintigraphy. Clin Nucl Med. 1987. 12:857–860.21. Zhang ZH, Wu SD, Wang B, Su Y, Jin JZ, Kong J, et al. Sphincter of Oddi hypomotility and its relationship with duodenal-biliary reflux, plasma motilin and serum gastrin. World J Gastroenterol. 2008. 14:4077–4081.22. Fein M, Bueter M, Sailer M, Fuchs KH. Effect of cholecystectomy on gastric and esophageal bile reflux in patients with upper gastrointestinal symptoms. Dig Dis Sci. 2008. 53:1186–1191.23. Arroyo AJ, Burns JB, Huyghe WA, Dollman AE, Patel YP. Enterogastric reflux mimicking gallbladder disease: detection, quantitation and potential significance. J Nucl Med Technol. 1999. 27:207–214.24. Kuran S, Parlak E, Aydog G, Kacar S, Sasmaz N, Ozden A, et al. Bile reflux index after therapeutic biliary procedures. BMC Gastroenterol. 2008. 8:4.25. Bordas JM, Elizalde I, Llach J, Mondelo F, Bataller R, Teres J. Biliary reflux due to sphincter of Oddi ablation: a new pathogenetic explanation for long-term major biliary symptoms after endoscopic-sphincterotomy. Endoscopy. 1996. 28:642.26. Bollschweiler E, Wolfgarten E, Putz B, Gutschow C, Holscher AH. Bile reflux into the stomach and the esophagus for volunteers older than 40 years. Digestion. 2005. 71:65–71.27. Byrne JP, Romagnoli R, Bechi P, Attwood SE, Fuchs KH, Collard JM. Duodenogastric reflux of bile in health: the normal range. Physiol Meas. 1999. 20:149–158.28. Koek GH, Sifrim D, Lerut T, Janssens J, Tack J. Multivariate analysis of the association of acid and duodeno-gastro-oesophageal reflux exposure with the presence of oesophagitis, the severity of oesophagitis and Barrett's oesophagus. Gut. 2008. 57:1056–1064.29. Kunsch S, Neesse A, Linhart T, Steinkamp M, Fensterer H, Adler G, et al. Impact of pantoprazole on duodeno-gastro-esophageal reflux (DGER). Z Gastroenterol. 2009. 47:277–282.30. Nakos A, Zezos P, Liratzopoulos N, Efraimidou E, Manolas K, Moschos J, et al. The significance of histological evidence of bile reflux gastropathy in patients with gastro-esophageal reflux disease. Med Sci Monit. 2009. 15:CR313–CR318.31. Myung SJ, Kim MH, Shim KN, Kim YS, Kim EO, Kim HJ, et al. Detection of Helicobacter pylori DNA in human biliary tree and its association with hepatolithiasis. Dig Dis Sci. 2000. 45:1405–1412.32. Stathopoulos P, Zundt B, Spelsberg FW, Kolligs L, Diebold J, Goke B, et al. Relation of gallbladder function and Helicobacter pylori infection to gastric mucosa inflammation in patients with symptomatic cholecystolithiasis. Digestion. 2006. 73:69–74.33. Oumi M, Yamamoto T. A scanning electron microscope study on the effects of different bile salts on the epithelial lining of jejunal mucosa. Med Electron Microsc. 2000. 33:11–15.34. van Malenstein H, Farre R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol. 2008. 103:1021–1028.35. Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Areni A, Scialpi C, et al. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003. 18:525–532.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Limy Bile Associated with Cholangitis and Calcified Gallbladder
- Endoscopic Management of Acute Cholecystitis and Cholangitis Caused by Limy Bile
- A clinical study of gallbladder and bile duct
- Unusual Presentation of Cystic Lymphangioma of the Gallbladder
- High pressure liquid chromatographic analysis of conjugated bile acids in gallbladder bile of Korean adults