Endocrinol Metab.
2015 Jun;30(2):221-225. 10.3803/EnM.2015.30.2.221.
A Calcitonin-Negative Neuroendocrine Tumor Derived from Follicular Lesions of the Thyroid
- Affiliations
-
- 1Department of Internal Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea. ejjeon@cu.ac.kr
- 2Department of Pathology, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 3Department of Otolaryngology Head and Neck Surgery, Catholic University of Daegu School of Medicine, Daegu, Korea.
- KMID: 2282014
- DOI: http://doi.org/10.3803/EnM.2015.30.2.221
Abstract
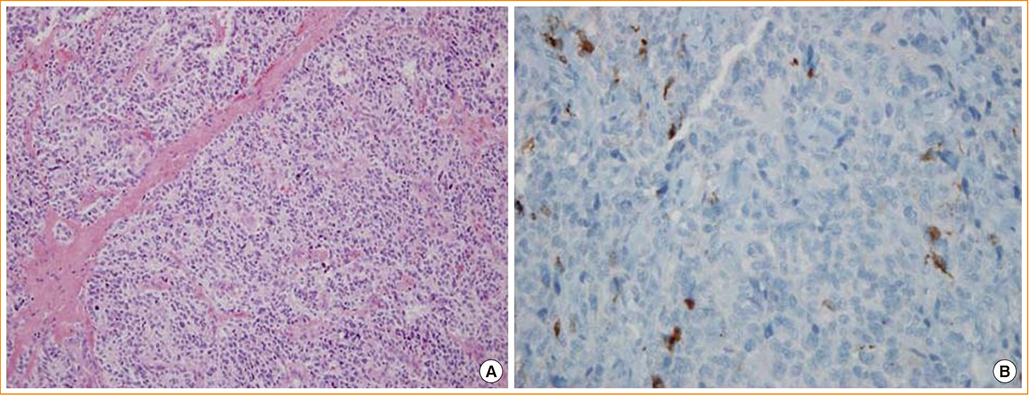
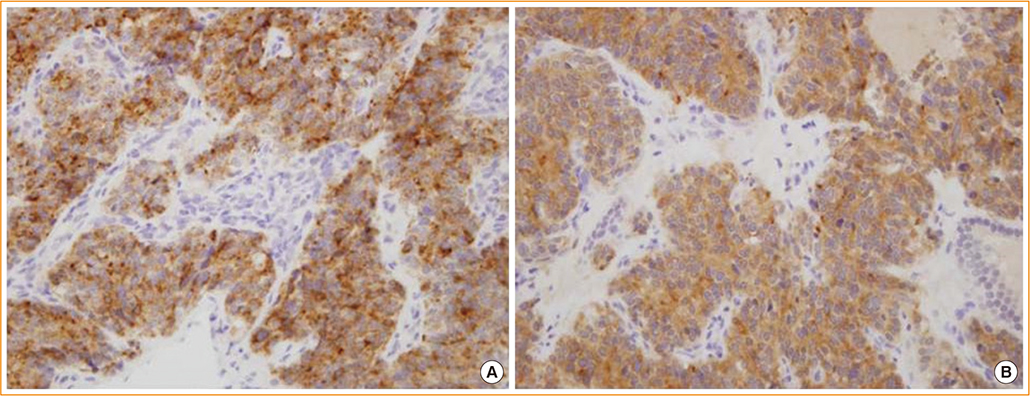
- Neuroendocrine lesions of the thyroid are rare. The most common types are medullary thyroid carcinomas (MTCs) and C-cell hyperplasia. MTCs originate from thyroid parafollicular cells that secrete calcitonin which serves as a serum marker of MTCs. Here, the rare case of a calcitonin-negative neuroendocrine tumor (NET) derived from follicular lesions of the thyroid is described. A 34-year-old man presented at our hospital for the surgical management of an incidental thyroid nodule that was observed on an ultrasound sonography (USG) of the neck. Initially, USG-guided aspiration cytology was performed, and a MTC was suspected. The expressions of thyroglobulin and thyroid transcription factor-1, which are thyroid follicular cell markers, and synaptophysin and chromogranin A, which are neuroendocrine markers, was confirmed following surgical pathology. However, the staining of calcitonin, a marker of MTCs, was not observed. A nonmedullary NET of the thyroid is uncommon, and the distinction between calcitonin-negative NETs and MTCs of the thyroid may be important due to differences in their clinical courses and management.
MeSH Terms
Figure
Reference
-
1. Baloch ZW, LiVolsi VA. Unusual tumors of the thyroid gland. Endocrinol Metab Clin North Am. 2008; 37:297–310.2. Kovaacs K, Asa SL. Chapter 13, The thyroid gland. Functional endocrine pathology. 2nd ed. Malden: Blackwell Science;1998. p. 295–380.3. Randolph GW. Surgery of the thyroid and parathyroid glands. 2nd ed. London: Elsevier Health Sciences;2012.4. Rosai J, Carcangiu ML, DeLellis RA. Armed Forces Institute of Pathology (US). Universities Associated for Research and Education in Pathology. Tumors of the thyroid gland. Washington DC: Armed Forces Institute of Pathology;1992.5. Griebeler ML, Gharib H, Thompson GB. Medullary thyroid carcinoma. Endocr Pract. 2013; 19:703–711.6. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Thyroid. 1995; 5:407–424.7. Marsh DJ, Learoyd DL, Robinson BG. Medullary thyroid carcinoma: recent advances and management update. Thyroid. 1995; 5:407–424.8. Almeida MQ, Stratakis CA. Solid tumors associated with multiple endocrine neoplasias. Cancer Genet Cytogenet. 2010; 203:30–36.9. Wells SA Jr, Dilley WG, Farndon JA, Leight GS, Baylin SB. Early diagnosis and treatment of medullary thyroid carcinoma. Arch Intern Med. 1985; 145:1248–1252.10. Bockhorn M, Frilling A, Rewerk S, Liedke M, Dirsch O, Schmid KW, Broelsch CE. Lack of elevated serum carcinoembryonic antigen and calcitonin in medullary thyroid carcinoma. Thyroid. 2004; 14:468–470.11. Chernyavsky VS, Farghani S, Davidov T, Ma L, Barnard N, Amorosa LF, Trooskin SZ. Calcitonin-negative neuroendocrine tumor of the thyroid: a distinct clinical entity. Thyroid. 2011; 21:193–196.12. Langley K. The neuroendocrine concept today. Ann N Y Acad Sci. 1994; 733:1–17.13. Baloch ZW, LiVolsi VA. Neuroendocrine tumors of the thyroid gland. Am J Clin Pathol. 2001; 115:S56–S67.14. American Thyroid, Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, Gharib H, Moley JF, Pacini F, Ringel MD, Schlumberger M, Wells SA Jr. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009; 19:565–612.15. Mussazhanova Z, Miura S, Stanojevic B, Rougounovitch T, Saenko V, Shiraishi T, Kurashige T, Shichijo K, Kaneko K, Takahashi H, Ito M, Nakashima M. Radiation-associated small cell neuroendocrine carcinoma of the thyroid: a case report with molecular analyses. Thyroid. 2014; 24:593–598.16. Elisei R, Bottici V, Luchetti F, Di Coscio G, Romei C, Grasso L, Miccoli P, Iacconi P, Basolo F, Pinchera A, Pacini F. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab. 2004; 89:163–168.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medullary Thyroid Carcinoma with Normal Calcitonin Level
- Immunohistochemical Analysis of Insular Carcinoma of the Thyroid Gland
- Composite Follicular Variant of Papillary Carcinoma and Mucoepidermoid Carcinoma of Thyroid Gland: A Case Report
- Recent Updates on the Management of Medullary Thyroid Carcinoma
- Cytologic Features of Medullary Carcinoma of the Thyroid Occurring in a Child: A Case Report