Diabetes Metab J.
2011 Aug;35(4):317-326. 10.4093/dmj.2011.35.4.317.
Polymeric Gene Delivery for Diabetic Treatment
- Affiliations
-
- 1Department of Pharmaceutics and Pharmaceutical Chemistry and Department of Bioengineering, University of Utah, Salt Lake City, UT, USA. SW.Kim@pharm.utah.edu
- 2Department of Bioengineering, Hanyang University, Seoul, Korea.
- KMID: 2281637
- DOI: http://doi.org/10.4093/dmj.2011.35.4.317
Abstract
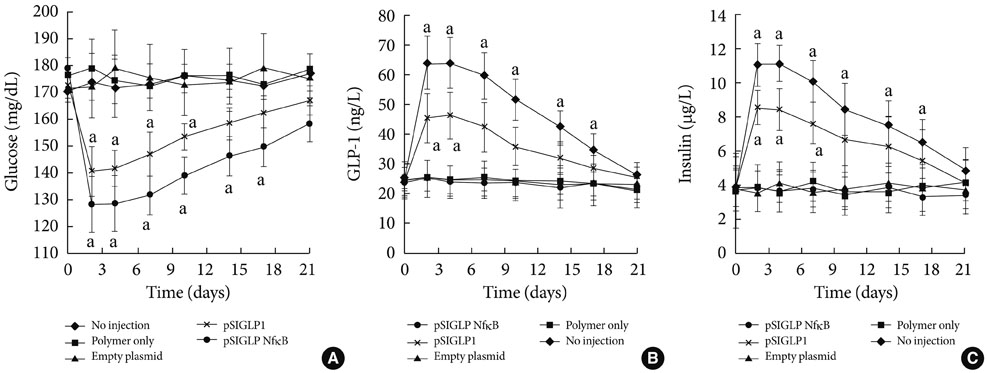
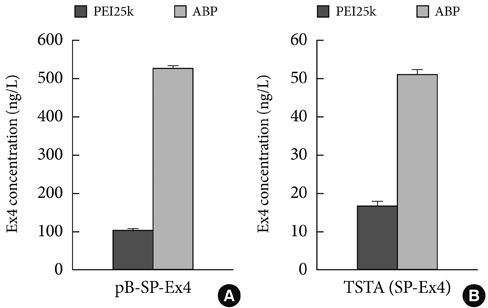
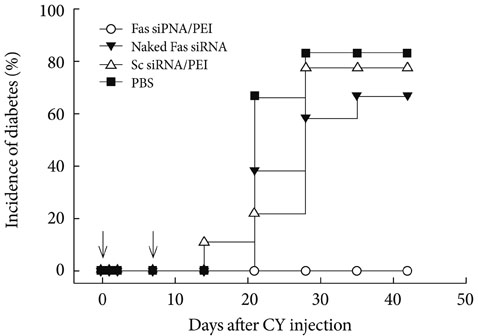
- Several polymers were used to delivery genes to diabetic animals. Polyaminobutyl glycolic acid was utilized to deliver IL-10 plasmid DNA to prevent autoimmune insulitis of non-obese diabetic (NOD) mouse. Polyethylene glycol grafted polylysine was combined with antisense glutamic acid decarboxylase (GAD) MRNA to represent GAD autoantigene expression. GLP1 and TSTA (SP-EX4) were delivered by bioreducible polymer to stop diabetic progression. Fas siRNA delivery was carried out to treat diabetic NOD mice animal.
Keyword
MeSH Terms
-
Animals
Antigens, Neoplasm
DNA
Glutamate Decarboxylase
Glycolates
Histocompatibility Antigens
Interleukin-10
Mice
Mice, Inbred NOD
Plasmids
Polyethylene Glycols
Polylysine
Polymers
RNA, Messenger
RNA, Small Interfering
Transplants
Antigens, Neoplasm
DNA
Glutamate Decarboxylase
Glycolates
Histocompatibility Antigens
Interleukin-10
Polyethylene Glycols
Polylysine
Polymers
RNA, Messenger
RNA, Small Interfering
Figure
Reference
-
1. Rolland AP. From genes to gene medicines: recent advances in nonviral gene delivery. Crit Rev Ther Drug Carrier Syst. 1998. 15:143–198.2. Peel D. Virus vectors and gene therapy: problems, promises and prospects. MBChB Special Study Module Project Report. 1998. Leicester: Department of Microbiology and Immunology, University of Leicester.3. Anchordoquy TJ, Koe GS. Physical stability of nonviral plasmid-based therapeutics. J Pharm Sci. 2000. 89:289–296.4. Garnett MC. Gene-delivery systems using cationic polymers. Crit Rev Ther Drug Carrier Syst. 1999. 16:147–207.5. Huang L, Hung M, Wagner E. Nonviral vectors for gene therapy. 1999. San Diego: Academic Press;3–22.6. Kawabata K, Takakura Y, Hashida M. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm Res. 1995. 12:825–830.7. American Diabetes Association. cited 2011 Jan 12. Available from: http://www.diabetes.org.8. Anderson R, Jones P. What's new in type 2 diabetes? 2000. Liverpool: National Prescribing Centre.9. Lorenzana GG, Hsia SH. The pharmacotherapy of type 2 diabetes to achieve tight glycemic control. Home Health Care Consult. 2002. 9:10–16.10. Malaisse WJ, Lebrun P. Mechanisms of sulfonylurea-induced insulin release. Diabetes Care. 1990. 13:Suppl 3. 9–17.11. Davies MJ. Insulin secretagogues. Curr Med Res Opin. 2002. 18:Suppl 1. s22–s30.12. Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001. 414:821–827.13. Koh JJ, Ko KS, Lee M, Han S, Park JS, Kim SW. Degradable polymeric carrier for the delivery of IL-10 plasmid DNA to prevent autoimmune insulitis of NOD mice. Gene Ther. 2000. 7:2099–2104.14. Lee M, Han S, Ko KS, Kim SW. Cell type specific and glucose responsive expression of interleukin-4 by using insulin promoter and water soluble lipopolymer. J Control Release. 2001. 75:421–429.15. Ko KS, Lee M, Koh JJ, Kim SW. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol Ther. 2001. 4:313–316.16. Lee M, Han SO, Ko KS, Koh JJ, Park JS, Yoon JW, Kim SW. Repression of GAD autoantigen expression in pancreas beta-Cells by delivery of antisense plasmid/PEG-g-PLL complex. Mol Ther. 2001. 4:339–346.17. Jeong JH, Lee M, Kim WJ, Yockman JW, Park TG, Kim YH, Kim SW. Anti-GAD antibody targeted non-viral gene delivery to islet beta cells. J Control Release. 2005. 107:562–570.18. La Barre J, Still EU. Studies on physiology of secretin. III. Further studies on the effects of secretin on blood sugar. Am J Physiol. 1930. 91:649–653.19. Creutzfeldt W, Ebert R. New developments in the incretin concept. Diabetologia. 1985. 28:565–573.20. Creutzfeldt W. The incretin concept today. Diabetologia. 1979. 16:75–85.21. Beck B. Gastric inhibitory polypeptide: a gut hormone with anabolic functions. J Mol Endocrinol. 1989. 2:169–174.22. Lund PK, Goodman RH, Montminy MR, Dee PC, Habener JF. Anglerfish islet pre-roglucagon II. Nucleotide and corresponding amino acid sequence of the cDNA. J Biol Chem. 1983. 258:3280–3284.23. Lopez LC, Frazier ML, Su CJ, Kumar A, Saunders GF. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proc Natl Acad Sci U S A. 1983. 80:5485–5489.24. MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002. 51:Suppl 3. S434–S442.25. Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care. 1992. 15:270–276.26. Buteau J, Roduit R, Susini S, Prentki M. Glucagon-like peptide-1 promotes DNA synthesis, activates phosphatidylinositol 3-kinase and increases transcription factor pancreatic and duodenal homeobox gene 1 (PDX-1) DNA binding activity in beta (INS-1)-cells. Diabetologia. 1999. 42:856–864.27. Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000. 141:4600–4605.28. Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995. 80:952–957.29. Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology. 2001. 142:521–527.30. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993. 36:741–744.31. Pridal L, Agerbaek H, Christensen LN, Thomsen K, Kirk O. Absorption of glucagons-like peptide-1 can be protracted by zinc or protamine. Int J Pharm. 1996. 136:53–59.32. Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998. 47:1663–1670.33. Oh S, Lee M, Ko KS, Choi S, Kim SW. GLP-1 gene delivery for the treatment of type 2 diabetes. Mol Ther. 2003. 7:478–483.34. Choi S, Oh S, Lee M, Kim SW. Glucagon-like peptide-1 plasmid construction and delivery for the treatment of type 2 diabetes. Mol Ther. 2005. 12:885–891.35. Jeong JH, Kim SH, Lee M, Kim WJ, Park TG, Ko KS, Kim SW. Non-viral systemic delivery of Fas siRNA suppresses cyclophosphamide-induced diabetes in NOD mice. J Control Release. 2010. 143:88–94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Polymeric Biomaterials for Drug Delivery System
- Mucosal Vaccine Delivery Using Mucoadhesive Polymer Particulate Systems
- Polymeric Gene Carriers and Their Applications to Diabetes Gene Therapy
- Development of chitosan-catechol conjugates as mucoadhesive polymer: assessment of acute oral toxicity in mice
- pH-sensitive Polymeric Micelles for the Effective Delivery of Anti-cancer Drug