Diabetes Metab J.
2012 Jun;36(3):199-206. 10.4093/dmj.2012.36.3.199.
Post-Renal Transplant Diabetes Mellitus in Korean Subjects: Superimposition of Transplant-Related Immunosuppressant Factors on Genetic and Type 2 Diabetic Risk Factors
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. endohclee@yuhs.ac
- KMID: 2281400
- DOI: http://doi.org/10.4093/dmj.2012.36.3.199
Abstract
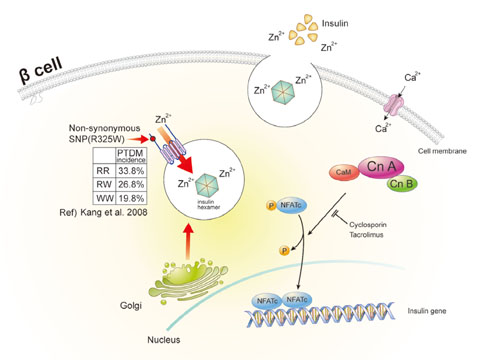
- Postrenal transplantation diabetes mellitus (PTDM), or new-onset diabetes after organ transplantation, is an important chronic transplant-associated complication. Similar to type 2 diabetes, decreased insulin secretion and increased insulin resistance are important to the pathophysiologic mechanism behind the development of PTDM. However, beta-cell dysfunction rather than insulin resistance seems to be a greater contributing factor in the development of PTDM. Increased age, family history of diabetes, ethnicity, genetic variation, obesity, and hepatitis C are partially accountable for an increased underlying risk of PTDM in renal allograft recipients. In addition, the use of and kinds of immunosuppressive agents are key transplant-associated risk factors. Recently, a number of genetic variants or polymorphisms susceptible to immunosuppressants have been reported to be associated with calcineurin inhibition-induced beta-cell dysfunction. The identification of high risk factors of PTDM would help prevent PTDM and improve long-term patient outcomes by allowing for personalized immunosuppressant regimens and by managing cardiovascular risk factors.
Keyword
MeSH Terms
Figure
Reference
-
1. Miles AM, Sumrani N, Horowitz R, Homel P, Maursky V, Markell MS, Distant DA, Hong JH, Sommer BG, Friedman EA. Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation. 1998. 65:380–384.2. Bloom RD, Crutchlow MF. New-onset diabetes mellitus in the kidney recipient: diagnosis and management strategies. Clin J Am Soc Nephrol. 2008. 3:Suppl 2. S38–S48.3. Starzl TE, Marchioro TL, Rifkind D, Holmes JH, Rowlands DT Jr, Waddell WR. Factors in successful renal transplantation. Surgery. 1964. 56:296–318.4. Crutchlow MF, Bloom RD. Transplant-associated hyperglycemia: a new look at an old problem. Clin J Am Soc Nephrol. 2007. 2:343–355.5. Hill CM, Douglas JF, Rajkumar KV, McEvoy J, McGeown MG. Glycosuria and hyperglycaemia after kidney transplantation. Lancet. 1974. 2:490–492.6. Arner P, Gunnarsson R, Blomdahl S, Groth CG. Some characteristics of steroid diabetes: a study in renal-transplant recipients receiving high-dose corticosteroid therapy. Diabetes Care. 1983. 6:23–25.7. Rao M, Jacob CK, Shastry JC. Post-renal transplant diabetes mellitus: a retrospective study. Nephrol Dial Transplant. 1992. 7:1039–1042.8. Ahn KJ, Kim YS, Lee HC, Park K, Huh KB. Clinical characteristics and possible risk factors in postrenal transplant diabetes mellitus. Transplant Proc. 1992. 24:1581–1582.9. Basri N, Aman H, Adiku W, Baraqdar A, Bonatero I, Nezamuddin N. Diabetes mellitus after renal transplantation. Transplant Proc. 1992. 24:1780–1781.10. Sumrani NB, Delaney V, Ding ZK, Davis R, Daskalakis P, Friedman EA, Butt KM, Hong JH. Diabetes mellitus after renal transplantation in the cyclosporine era: an analysis of risk factors. Transplantation. 1991. 51:343–347.11. von Kiparski A, Frei D, Uhlschmid G, Largiader F, Binswanger U. Post-transplant diabetes mellitus in renal allograft recipients: a matched-pair control study. Nephrol Dial Transplant. 1990. 5:220–225.12. Lee HC, Nam MS, Nam SY, Cha BS, Lee JH, Song YD, Lee EJ, Lim SK, Kim KR, Kim YS, Park K, Huh KB. Posttransplant diabetes mellitus after renal transplantation in Korea. Transplant Proc. 1996. 28:1159–1160.13. Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003. 3:590–598.14. Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003. 3:178–185.15. Hur KY, Kim MS, Kim YS, Kang ES, Nam JH, Kim SH, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC. Risk factors associated with the onset and progression of posttransplantation diabetes in renal allograft recipients. Diabetes Care. 2007. 30:609–615.16. Hjelmesaeth J, Hartmann A, Kofstad J, Stenstrom J, Leivestad T, Egeland T, Fauchald P. Glucose intolerance after renal transplantation depends upon prednisolone dose and recipient age. Transplantation. 1997. 64:979–983.17. Rodrigo E, Fernandez-Fresnedo G, Valero R, Ruiz JC, Pinera C, Palomar R, Gonzalez-Cotorruelo J, Gomez-Alamillo C, Arias M. New-onset diabetes after kidney transplantation: risk factors. J Am Soc Nephrol. 2006. 17:12 Suppl 3. S291–S295.18. Markell M. New-onset diabetes mellitus in transplant patients: pathogenesis, complications, and management. Am J Kidney Dis. 2004. 43:953–965.19. Lanerolle RD, de Abrew K, Fernando DJ, Sheriff MH. Post-renal transplant diabetes in Sri Lanka. Transplant Proc. 1996. 28:1945–1947.20. Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002. 13:1374–1380.21. Nam JH, Mun JI, Kim SI, Kang SW, Choi KH, Park K, Ahn CW, Cha BS, Song YD, Lim SK, Kim KR, Lee HC, Huh KB. beta-Cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation. 2001. 71:1417–1423.22. Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004. 4:583–595.23. Ekstrand AV, Eriksson JG, Gronhagen-Riska C, Ahonen PJ, Groop LC. Insulin resistance and insulin deficiency in the pathogenesis of posttransplantation diabetes in man. Transplantation. 1992. 53:563–569.24. Midtvedt K, Hartmann A, Hjelmesaeth J, Lund K, Bjerkely BL. Insulin resistance is a common denominator of post-transplant diabetes mellitus and impaired glucose tolerance in renal transplant recipients. Nephrol Dial Transplant. 1998. 13:427–431.25. Shimizu M, Iino Y, Terashi A. Improvement of insulin sensitivity after renal transplantation measured by a glucose clamp technique. Nihon Ika Daigaku Zasshi. 1998. 65:50–54.26. Hagen M, Hjelmesaeth J, Jenssen T, Morkrid L, Hartmann A. A 6-year prospective study on new onset diabetes mellitus, insulin release and insulin sensitivity in renal transplant recipients. Nephrol Dial Transplant. 2003. 18:2154–2159.27. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001. 50:590–593.28. Rhee SY, Woo JT. The prediabetic period: review of clinical aspects. Diabetes Metab J. 2011. 35:107–116.29. Kim I, Kang ES, Yim YS, Ko SJ, Jeong SH, Rim JH, Kim YS, Ahn CW, Cha BS, Lee HC, Kim CH. A low-risk ZnT-8 allele (W325) for post-transplantation diabetes mellitus is protective against cyclosporin A-induced impairment of insulin secretion. Pharmacogenomics J. 2011. 11:191–198.30. Nielsen JH, Mandrup-Poulsen T, Nerup J. Direct effects of cyclosporin A on human pancreatic beta-cells. Diabetes. 1986. 35:1049–1052.31. Robertson RP. Cyclosporin-induced inhibition of insulin secretion in isolated rat islets and HIT cells. Diabetes. 1986. 35:1016–1019.32. Paty BW, Harmon JS, Marsh CL, Robertson RP. Inhibitory effects of immunosuppressive drugs on insulin secretion from HIT-T15 cells and Wistar rat islets. Transplantation. 2002. 73:353–357.33. Polastri L, Galbiati F, Bertuzzi F, Fiorina P, Nano R, Gregori S, Aldrighetti L, Pozza G, Secchi A, Adorini L, Davalli AM. Secretory defects induced by immunosuppressive agents on human pancreatic beta-cells. Acta Diabetol. 2002. 39:229–233.34. Redmon JB, Olson LK, Armstrong MB, Greene MJ, Robertson RP. Effects of tacrolimus (FK506) on human insulin gene expression, insulin mRNA levels, and insulin secretion in HIT-T15 cells. J Clin Invest. 1996. 98:2786–2793.35. Uchizono Y, Iwase M, Nakamura U, Sasaki N, Goto D, Iida M. Tacrolimus impairment of insulin secretion in isolated rat islets occurs at multiple distal sites in stimulus-secretion coupling. Endocrinology. 2004. 145:2264–2272.36. Lawrence MC, Bhatt HS, Watterson JM, Easom RA. Regulation of insulin gene transcription by a Ca(2+)-responsive pathway involving calcineurin and nuclear factor of activated T cells. Mol Endocrinol. 2001. 15:1758–1767.37. Oetjen E, Baun D, Beimesche S, Krause D, Cierny I, Blume R, Dickel C, Wehner S, Knepel W. Inhibition of human insulin gene transcription by the immunosuppressive drugs cyclosporin A and tacrolimus in primary, mature islets of transgenic mice. Mol Pharmacol. 2003. 63:1289–1295.38. Lawrence MC, Bhatt HS, Easom RA. NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes. 2002. 51:691–698.39. Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006. 443:345–349.40. Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH. Sirolimus is associated with reduced islet engraftment and impaired beta-cell function. Diabetes. 2006. 55:2429–2436.41. Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, Leibowitz G. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008. 57:945–957.42. Veroux M, Corona D, Giuffrida G, Gagliano M, Sorbello M, Virgilio C, Tallarita T, Zerbo D, Giaquinta A, Fiamingo P, Macarone M, Li Volti G, Caglia P, Veroux P. New-onset diabetes mellitus after kidney transplantation: the role of immunosuppression. Transplant Proc. 2008. 40:1885–1887.43. Shimodahira M, Fujimoto S, Mukai E, Nakamura Y, Nishi Y, Sasaki M, Sato Y, Sato H, Hosokawa M, Nagashima K, Seino Y, Inagaki N. Rapamycin impairs metabolism-secretion coupling in rat pancreatic islets by suppressing carbohydrate metabolism. J Endocrinol. 2010. 204:37–46.44. Heaton DA, Millward BA, Gray IP, Tun Y, Hales CN, Pyke DA, Leslie RD. Increased proinsulin levels as an early indicator of B-cell dysfunction in non-diabetic twins of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1988. 31:182–184.45. Roder ME, Porte D Jr, Schwartz RS, Kahn SE. Disproportionately elevated proinsulin levels reflect the degree of impaired B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998. 83:604–608.46. Sumrani N, Delaney V, Ding Z, Davis R, Daskalakis P, Friedman EA, Butt KM, Hong JH. Posttransplant diabetes mellitus in cyclosporine-treated renal transplant recipients. Transplant Proc. 1991. 23(1 Pt 2):1249–1250.47. Tavira B, Coto E, Torres A, Diaz-Corte C, Diaz-Molina B, Ortega F, Arias M, Diaz JM, Selgas R, Lopez-Larrea C, Ruiz-Ortega M, Ortiz A, Gonzalez E, Campistol JM, Alvarez V. The Pharmacogenetics of tacrolimus REDINREN study group. Association between a common KCNJ11 polymorphism (rs5219) and new-onset posttransplant diabetes in patients treated with Tacrolimus. Mol Genet Metab. 2012. 105:525–527.48. Yang J, Hutchinson II, Shah T, Min DI. Genetic and clinical risk factors of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation. 2011. 91:1114–1119.49. Chen Y, Sampaio MS, Yang JW, Min D, Hutchinson IV. Genetic polymorphisms of the transcription factor NFATc4 and development of new-onset diabetes after transplantation in Hispanic kidney transplant recipients. Transplantation. 2012. 93:325–330.50. Szuszkiewicz M, Bell J, Vazquez M, Adams-Huet B, Grundy SM, Chandalia M, Abate N. ENPP1/PC-1 K121Q and other predictors of posttransplant diabetes. Metab Syndr Relat Disord. 2011. 9:25–29.51. Jeong KH, Moon JY, Chung JH, Kim YH, Lee TW. Significant associations between CCL5 gene polymorphisms and post-transplantational diabetes mellitus in Korean renal allograft recipients. Am J Nephrol. 2010. 32:356–361.52. Numakura K, Satoh S, Tsuchiya N, Horikawa Y, Inoue T, Kakinuma H, Matsuura S, Saito M, Tada H, Suzuki T, Habuchi T. Clinical and genetic risk factors for posttransplant diabetes mellitus in adult renal transplant recipients treated with tacrolimus. Transplantation. 2005. 80:1419–1424.53. Kang ES, Magkos F, Kim BS, Zhai R, Su L, Kim YS, Christiani DC, Lee HC, Mantzoros CS. Variants of the adiponectin and adiponectin receptor-1 genes and posttransplantation diabetes mellitus in renal allograft recipients. J Clin Endocrinol Metab. 2012. 97:E129–E135.54. Yu SJ, Peng L, Xie XB, Peng FH, Fang CH, Wang Y, Lan GB. Correlation between HLA and posttransplantation diabetes mellitus in the Han population in South China. Transplant Proc. 2010. 42:2509–2512.55. Chang HR, Yang SF, Tsai JP, Hsieh MC, Wu SW, Tsai HC, Hung TW, Huang JH, Lian JD. Plasminogen activator inhibitor-1 5G/5G genotype is a protecting factor preventing posttransplant diabetes mellitus. Clin Chim Acta. 2011. 412:322–326.56. Tsai JP, Yang SF, Wu SW, Hung TW, Tsai HC, Lian JD, Chang HR. Glutathione S-transferase gene polymorphisms are not major risks for susceptibility to posttransplantation diabetes mellitus in Taiwan renal transplant recipients. J Clin Lab Anal. 2011. 25:432–435.57. Dutkiewicz G, Domanski L, Pawlik A, Binczak-Kuleta A, Safranow K, Ciechanowicz A, Dziedziejko V, Pietrzak-Nowacka M, Ciechanowski K. Polymorphisms of superoxide dismutase, glutathione peroxidase and catalase genes in patients with post-transplant diabetes mellitus. Arch Med Res. 2010. 41:350–355.58. Kang ES, Kim MS, Kim YS, Hur KY, Han SJ, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC. A variant of the transcription factor 7-like 2 (TCF7L2) gene and the risk of posttransplantation diabetes mellitus in renal allograft recipients. Diabetes Care. 2008. 31:63–68.59. Kang ES, Kim MS, Kim YS, Kim CH, Han SJ, Chun SW, Hur KY, Nam CM, Ahn CW, Cha BS, Kim SI, Lee HC. A polymorphism in the zinc transporter gene SLC30A8 confers resistance against posttransplantation diabetes mellitus in renal allograft recipients. Diabetes. 2008. 57:1043–1047.60. Kang ES, Kim MS, Kim CH, Nam CM, Han SJ, Hur KY, Ahn CW, Cha BS, Kim SI, Lee HC, Kim YS. Association of common type 2 diabetes risk gene variants and posttransplantation diabetes mellitus in renal allograft recipients in Korea. Transplantation. 2009. 88:693–698.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Posttransplant Diabetes Mellitus
- Pancreas transplant in type 1 diabetes mellitus: the emerging role of islet cell transplant
- Clinical Presentation of Diabetes Mellitus after Kidney Tansplantation
- Post-renal Transplant Diabetes Mellitus-a Retrospective Study
- Post-transplant Diabetic Ketoacidosis-two Cases