Clin Exp Otorhinolaryngol.
2010 Mar;3(1):13-17.
Effect of Nebulized Bovine Surfactant for Experimental Otitis Media with Effusion
- Affiliations
-
- 1Department of Otolaryngology, Chonnam National University Medical School, Gwangju, Korea. chulsavio@hanmail.net
- 2Research Center for Resistant Cells, Chosun University Medical School, Gwangju, Korea.
Abstract
OBJECTIVES
In this study, we evaluated the efficacy of nebulized bovine pulmonary surfactant on experimentally induced otitis media with effusion (OME) in guinea pigs.
METHODS
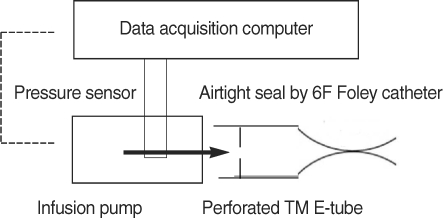
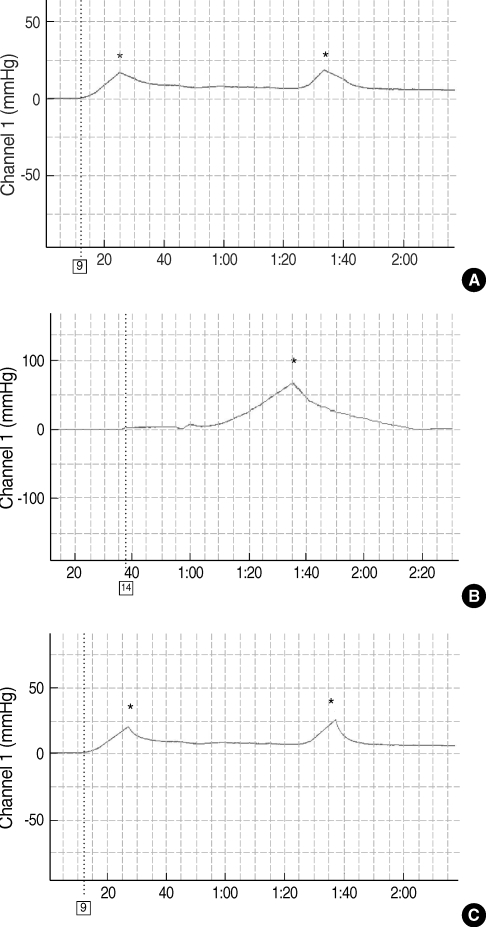
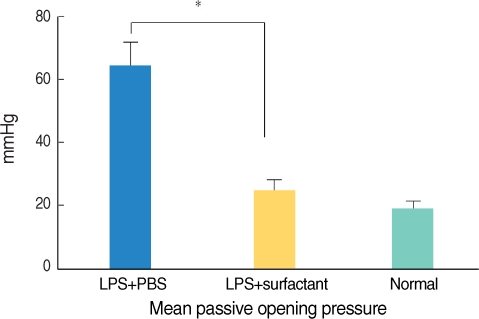
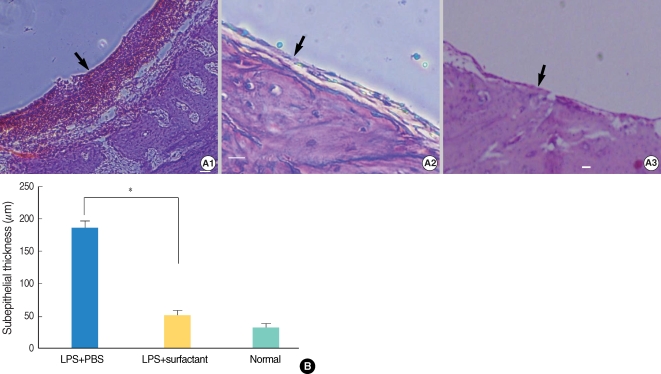
Twenty guinea pigs were divided into three groups. Four untreated animals served as normal controls. Experimental OME was established in both ears of the remaining 16 animals by a transbullar injection of 10 microL of Pseudomonas aeruginosa lipopolysaccharide in saline. Thereafter, the guinea pigs received nebulized phosphate buffered saline (n=8) or nebulized bovine pulmonary surfactant (n=8). Nebulization was given daily for 7 days. On day 8, all the animals' passive opening pressure (POP) of the Eustachian tube was measured and histopathological observations of the bulla were made by light microscopy.
RESULTS
Nebulized bovine pulmonary surfactant significantly reduced the POP compared to that of saline nebulization. The bovine pulmonary surfactant improved the tubal patency and produced less histopathologcally-evident edematous bullar mucosa.
CONCLUSION
Nebulization of bovine pulmonary surfactant plays an important role in treating otitis media with effusion in guinea pigs. Our results suggest that the chosen nebulized bovine pulmonary surfactant can be of good clinical benefit for treating OME in the future.
MeSH Terms
Figure
Reference
-
1. Bluestone CD. Eustachian tube function: physiology, pathophysiology, and role of allergy in pathogenesis of otitis media. J Allergy Clin Immunol. 1983; 9. 72(3):242–251. PMID: 6886258.
Article2. Flisberg K, Ingelstedt S, Ortegren U. The valve and "locking" mechanisms of the eustachian tube. Acta Otolaryngol Suppl. 1963; 182:57–68.
Article3. Birken EA, Brookler KH. Surface tension lowering substance of the canine Eustachian tube. Ann Otol Rhinol Laryngol. 1972; 4. 81(2):268–271. PMID: 5067550.
Article4. Rapport PN, Lim DJ, Weiss HS. Surface-active agent in Eustachian Tube Function. Arch Otolaryngol. 1975; 5. 101(5):305–311. PMID: 1131089.
Article5. Maves MD, Patil GS, Lim DJ. Surface-active substances of the guinea pig tubotympanum: a chemical and physical analysis. Otolaryngol Head Neck Surg. 1981; Mar–Apr. 89(2):307–316. PMID: 6787533.
Article6. Grace A, Kwok P, Hawke M. Surfactant in middle ear effusions. Otolaryngol Head Neck Surg. 1987; 4. 96(4):336–340. PMID: 3108821.
Article7. White P, Hermansson A, Svinhufvud M. Surfactant and isoprenaline effect on eustachian tube opening in rats with acute otitis media. Am J Otolaryngol. 1990; Nov–Dec. 11(6):389–392. PMID: 2281840.
Article8. Nemechek AJ, Pahlavan N, Cote DN. Nebulized surfactant for experimentally induced otitis media with effusion. Otolaryngol Head Neck Surg. 1997; 11. 117(5):475–479. PMID: 9374170.
Article9. Koten M, Uzun C, Yagiz R, Adali MK, Karasalihoglu AR, Tatman-Otkun M, et al. Nebulized surfactant as a treatment choice for otitis media with effusion: an experimental study in the rabbit. J Laryngol Otol. 2001; 5. 115(5):363–368. PMID: 11410125.
Article10. Venkatayan N, Troublefield YL, Connelly PE, Mautone AJ, Chandrasekhar SS. Intranasal surfactant aerosol therapy for otitis media with effusion. Laryngoscope. 2000; 11. 110(11):1857–1860. PMID: 11081599.
Article11. Chandrasekhar SS, Connelly PE, Venkatayan N, el-Sherif Ammar M, Tabor M, Mautone AJ. Intranasal metered dose aerosolized surfactant reduces passive opening pressure of the eustachian tube: comparison study in two animal models. Otol Neurotol. 2002; 1. 23(1):3–7. PMID: 11773836.
Article12. Chandrasekhar SS, Mautone AJ. Otitis media: treatment with intranasal aerosolized surfactant. Laryngoscope. 2004; 3. 114(3):472–485. PMID: 15091221.
Article13. Tegtmeyer FK, Gortner L, Ludwig A, Brandt E. In vitro modulation of induced neutrophil activation by different surfactant preparations. Eur Respir J. 1996; 4. 9(4):752–757. PMID: 8726941.14. Reiss I, Kuntz S, Schmidt R, Kunz C, Gortner L, Rudloff S. Effect of pulmonary surfactant on TNF-alpha-activated endothelial cells and neutrophil adhesion in vitro. Immunobiology. 2004; 209(3):235–244. PMID: 15518335.15. Chabot S, Salez L, McCormack FX, Touqui L, Chignard M. Surfactant protein A inhibits lipopolysaccharide-induced in vivo production of interleukin-10 by mononuclear phagocytes during lung inflammation. Am J Respir Cell Mol Biol. 2003; 3. 28(3):347–353. PMID: 12594061.16. Tooley WH, Clements JA, Muramatsu K, Brown CL, Schlueter MA. Lung function in prematurely delivered rabbits treated with a synthetic surfactant. Am Rev Respir Dis. 1987; 9. 136(3):651–656. PMID: 3307569.
Article17. Counter SA, Borg E, Engstrom B. Acoustic middle ear reflexes in laboratory animals using clinical equipment: technical considerations. Audiology. 1989; 28(3):135–143. PMID: 2735848.
Article18. Moller AR. An experimental study of the acoustic impedance of the middle ear and its transmission properties. Acta Otolaryngol. 1965; Jul–Aug. 60(1):129–149. PMID: 14337949.19. Daniel HJ 3rd, Fulghum RS, Brinn JE, Barrett KA. Comparative anatomy of eustachian tube and middle ear cavity in animal models for otitis media. Ann Otol Rhinol Laryngol. 1982; Jan–Feb. 91(1 Pt 1):82–89. PMID: 7073182.
Article20. Tos M, Bak-Pedersen K. Goblet cell population in the pathological middle ear and eustachian tube of children and adults. Ann Otol Rhinol Laryngol. 1977; Mar–Apr. 86(2 Pt 1):209–218. PMID: 848832.
Article21. Yoon YJ, Jeong HS, Kim YK, Lee BK. The distribution of elastic fibers in the Eustachian tube of the rat. Korean J Otolaryngol-Head Neck Surg. 1996; 4. 39(4):568–575.22. Kabha K, Schmegner J, Keisari Y, Parolis H, Schlepper-Schaeffer J, Ofek I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol. 1997; 2. 272(2 Pt 1):L344–L352. PMID: 9124386.
Article23. McNeely TB, Coonrod JD. Comparison of the opsonic activity of human surfactant protein A for Staphylococcus aureus and Streptococcus pneumoniae with rabbit and human macrophages. J Infect Dis. 1993; 1. 167(1):91–97. PMID: 8418186.
Article24. Ofek I, Mesika A, Kalina M, Keisari Y, Podschun R, Sahly H, et al. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect Immun. 2001; 1. 69(1):24–33. PMID: 11119485.25. Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, et al. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003; 5. 111(10):1589–1602. PMID: 12750409.
Article26. Ryan MA, Akinbi HT, Serrano AG, Perez-Gil J, Wu H, McCormack FX, et al. Antimicrobial activity of native and synthetic surfactant protein B peptides. J Immunol. 2006; 1. 176(1):416–425. PMID: 16365435.
Article27. Kondo H, Suzuki K, Takagi I, Miyamoto N, Baba S, Kobayashi T, et al. Transitional concentration of antibacterial agent to the maxillary sinus via a nebuliser. Acta Otolaryngol Suppl. 1996; 525:64–67. PMID: 8908273.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Experimental otitis media with effusion induced by lipopolysaccharides from E. coli: the effects of endotoxin to the chronically of OME
- Development of Animal Models of Otitis Media
- Allele Distribution of Human Surfactant Protein A in Otitis Media with Effusion
- Middle ear histopathology in children with otitis media with effusion
- Prognostic significance of mastoid pneumatization in childhood otitis media with effusion: temporal bone CT evaluation