Intravenous Nefopam Reduces Postherpetic Neuralgia during the Titration of Oral Medications
- Affiliations
-
- 1Department of Anesthesia and Pain Medicine, School of Medicine, Pusan National University, Yangsan, Korea. pain@pusan.ac.kr
- 2Department of Plastic Surgery, School of Medicine, Dongguk University, Gyeongju, Korea.
- KMID: 2278203
- DOI: http://doi.org/10.3344/kjp.2014.27.1.54
Abstract
- BACKGROUND
The recently known analgesic action mechanisms of nefopam (NFP) are similar to those of anticonvulsants and antidepressants in neuropathic pain treatment. It is difficult to prescribe high doses of oral neuropathic drugs without titration due to adverse effects. Unfortunately, there are few available intravenous analgesics for the immediate management of acute flare-ups of the chronic neuropathic pain. The aim of this study was to determine the additional analgesic effects for neuropathic pain of NFP and its adverse effects during the titration of oral medications for neuropathic pain among inpatients with postherpetic neuralgia (PHN).
METHODS
Eighty inpatients with PHN were randomly divided into either the NFP or normal saline (NS) groups. Each patient received a 3-day intravenous continuous infusion of either NFP with a consecutive dose reduction of 60, 40, and 20 mg/d, or NS simultaneously while dose titrations of oral medications for neuropathic pain gradually increased every 3 days. The efficacy of additional NFP was evaluated by using the neuropathic pain symptom inventory (NPSI) score for 12 days. Adverse effects were also recorded.
RESULTS
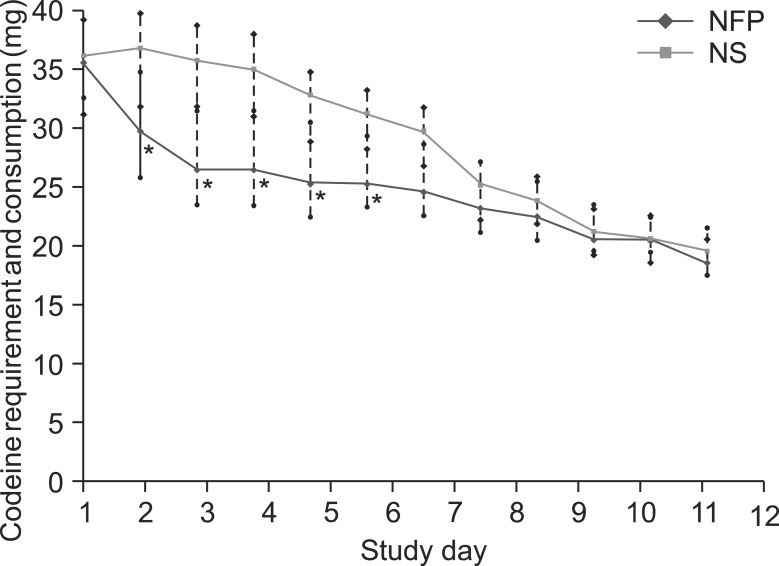
The median NPSI score was significantly lower in the NFP group from days 1 to 6 of hospitalization. The representative alleviating symptoms of pain after using NFP were both spontaneous and evoked neuropathic pain. Reported common adverse effects were nausea, dizziness, and somnolence, in order of frequency.
CONCLUSIONS
An intravenous continuous infusion of NFP reduces spontaneous and evoked neuropathic pain with tolerable adverse effects during the titration of oral medications in inpatients with PHN.
MeSH Terms
Figure
Cited by 4 articles
-
Medications in Treatment of Postherpetic Neuralgia
Sang Wook Shin
Korean J Pain. 2014;27(1):1-2. doi: 10.3344/kjp.2014.27.1.1.Nefopam Reduces Dysesthesia after Percutaneous Endoscopic Lumbar Discectomy
Young Min Ok, Ji Hyun Cheon, Eun Ji Choi, Eun Jung Chang, Ho Myung Lee, Kyung Hoon Kim
Korean J Pain. 2016;29(1):40-47. doi: 10.3344/kjp.2016.29.1.40.Use of Nefopam in Perioperative Pain Management; Keeping Nefopam in between
Jeong Il Choi
Korean J Pain. 2016;29(2):71-72. doi: 10.3344/kjp.2016.29.2.71.Earlier treatment improves the chances of complete relief from postherpetic neuralgia
Dong Hee Kang, Su Young Kim, Hyuck Goo Kim, Jung Hyun Park, Tae Kyun Kim, Kyung Hoon Kim
Korean J Pain. 2017;30(3):214-219. doi: 10.3344/kjp.2017.30.3.214.
Reference
-
1. Turk DC, Okifuji A. Pain terms and taxonomies. In : Fishman SM, Ballantyne JC, Rathmell JP, editors. Bonica's management of pain. 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins;2010. p. 16.2. Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS. Nefopam: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1980; 19:249–267. PMID: 6991238.3. Novelli A, Díaz-Trelles R, Groppetti A, Fernández-Sánchez MT. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids. 2005; 28:183–191. PMID: 15714253.
Article4. Verleye M, André N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004; 1013:249–255. PMID: 15193535.
Article5. Biella GE, Groppetti A, Novelli A, Fernández-Sánchez MT, Manfredi B, Sotgiu ML. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma. 2003; 20:593–601. PMID: 12906743.
Article6. Novelli A, Groppetti A, Rossoni G, Manfredi B, Ferrero-Gutiérrez A, Pérez-Gómez A, et al. Nefopam is more potent than carbamazepine for neuroprotection against veratridine in vitro and has anticonvulsant properties against both electrical and chemical stimulation. Amino Acids. 2007; 32:323–332. PMID: 17021653.
Article7. Czuczwar M, Czuczwar K, Cięszczyk J, Kiś J, Saran T, Łuszczki JJ, et al. Nefopam enhances the protective activity of antiepileptics against maximal electroshock-induced convulsions in mice. Pharmacol Rep. 2011; 63:690–696. PMID: 21857079.
Article8. Löscher W, Schmidt D. New Horizons in the development of antiepileptic drugs: Innovative strategies. Epilepsy Res. 2006; 69:183–272. PMID: 16835945.
Article9. Marazziti D, Rotondo A, Ambrogi F, Cassano GB. Analgesia by nefopam: does it act through serotonin? Drugs Exp Clin Res. 1991; 17:259–261. PMID: 1756689.10. Evans MS, Lysakowski C, Tramèr MR. Nefopam for the prevention of postoperative pain: quantitative systematic review. Br J Anaesth. 2008; 101:610–617. PMID: 18796441.
Article11. Sunshine A, Laska E. Nefopam and morphine in man. Clin Pharmacol Ther. 1975; 18:530–534. PMID: 1102231.
Article12. Taniguchi Y, Ali SZ, Kimberger O, Zmoos S, Lauber R, Markstaller M, et al. The effects of nefopam on the gain and maximum intensity of shivering in healthy volunteers. Anesth Analg. 2010; 111:409–414. PMID: 20529984.
Article13. Alfonsi P, Passard A, Gaude-Joindreau V, Guignard B, Sessler DI, Chauvin M. Nefopam and alfentanil additively reduce the shivering threshold in humans whereas nefopam and clonidine do not. Anesthesiology. 2009; 111:102–109. PMID: 19512866.
Article14. Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004; 108:248–257. PMID: 15030944.
Article15. Thakur R, Kent JL, Dworkin RH. Herpes zoster and postherpetic neuralgia. In : Fishman SM, Ballantyne JC, Rathmell JP, editors. Bonica's management of pain. 4th ed. Philadelphia (PA): Lippincott Williams & Wilkins;2010. p. 338–357.16. Magrinelli F, Zanette G, Tamburin S. Neuropathic pain: diagnosis and treatment. Pract Neurol. 2013; 13:292–307. PMID: 23592730.
Article17. Attal N. Neuropathic pain: mechanisms, therapeutic approach, and interpretation of clinical trials. Continuum (Minneap Minn). 2012; 18:161–175. PMID: 22810075.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Trial of Continuous Intravenous Infusion of Magnesium in Patients with Postherpetic Neuralgia Refractory in Conventional Treatment: A report of 2 cases
- Treatment of Herpes Zoster: Nerve Block
- Medications in Treatment of Postherpetic Neuralgia
- The Effects of Continuous Epidural Blockade in the Treatment of Postherpetic Neuralgia
- Stuttering during Management of Postherpetic Neuralgia: A case report