Korean J Obstet Gynecol.
2012 Nov;55(11):814-821. 10.5468/KJOG.2012.55.11.814.
Synergistic effects of sorafenib and celecoxib inhibit growth and VEGF expression in Hec-1A endometrial cancer cell line
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Soonchunhyang Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea. jskim@schmc.ac.kr
- 2Department of Obstetrics and Gynecology, Soonchunhyang Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 3Department of Obstetrics and Gynecology, Soonchunhyang Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea.
- KMID: 2274149
- DOI: http://doi.org/10.5468/KJOG.2012.55.11.814
Abstract
OBJECTIVE
The aim of this study was to investigate whether combination of sorafenib and celecoxib exhibited an anti-tumor efficacy or altered expression of vascular endothelial growth factor (VEGF) in Hec-1A endometrial cancer cell line.
METHODS
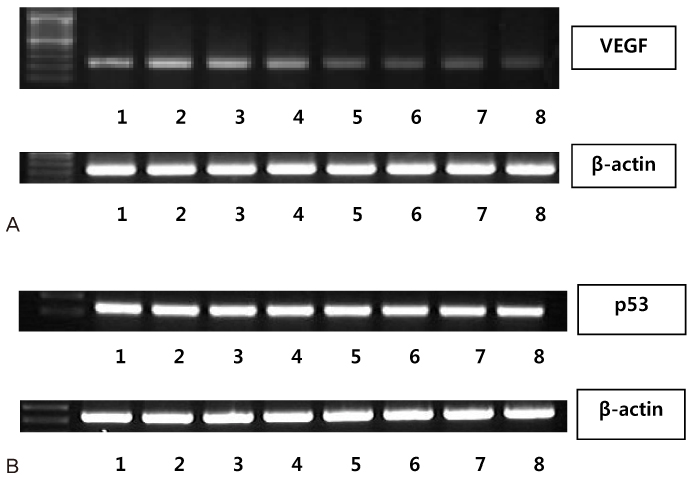
To determine whether sorafenib or celecoxib-induced growth inhibition was determined by the (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium, inner salt) assay. Expression of VEGF and p53 were evaluated using the reverse transcription polymerase chain reaction.
RESULTS
Combination of sorafenib 10 ng/mL and celecoxib 50 micromol/L exhibited synergistic inhibitory effects compared to treatment with each agent alone (P<0.0001). VEGF expression was also down regulated after 24 hours or 72 hours of treatment with sorafenib alone or in combination with sorafenib and celecoxib in Hec-1A cells. However, there was no alteration of p53 expression in Hec-1A cells after 24 hours or 72 hours of treatment with sorafenib alone or in combination with sorafenib and celecoxib.
CONCLUSION
Combination treatment of sorafenib and celecoxib to Hec-1A endometrial cancer cell line revealed the ability to inhibit growth and expression of VEGF.
MeSH Terms
Figure
Reference
-
1. SOG Gynecologic Oncology Committee, Korean Society of Obstetrics and Gynecology. Annual report of gynecologic cancer registry program in Korea for 2004 (Jan. 1st, 2004-Dec. 31st, 2004). Korean J Obstet Gynecol. 2007. 50:28–78.2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.3. Kim JW, Kim SH, Kim YT, Kim DK. Clinicopathologic and biological parameters predicting the prognosis in endometrial cancer. Yonsei Med J. 2002. 43:769–778.4. Lee SE, Kim JW, Park NH, Song YS, Kang SB, Lee HP. Contemporary trends of endometrial cancer in Korean women. Korean J Gynecol Oncol. 2005. 16:215–220.5. Cohn DE, Horowitz NS, Mutch DG, Kim SM, Manolitsas T, Fowler JM. Should the presence of lymphvascular space involvement be used to assign patients to adjuvant therapy following hysterectomy for unstaged endometrial cancer? Gynecol Oncol. 2002. 87:243–246.6. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991. 40:55–65.7. Mariani A, Webb MJ, Keeney GL, Aletti G, Podratz KC. Endometrial cancer: predictors of peritoneal failure. Gynecol Oncol. 2003. 89:236–242.8. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.9. Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992. 3:65–71.10. Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985. 43:175–203.11. Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987. 235:442–447.12. Maeda K, Kang SM, Ogawa M, Onoda N, Sawada T, Nakata B, et al. Combined analysis of vascular endothelial growth factor and platelet-derived endothelial cell growth factor expression in gastric carcinoma. Int J Cancer. 1997. 74:545–550.13. Malden LT, Novak U, Burgess AW. Expression of transforming growth factor alpha messenger RNA in the normal and neoplastic gastro-intestinal tract. Int J Cancer. 1989. 43:380–384.14. Chung CK, Antoniades HN. Expression of c-sis/platelet-derived growth factor B, insulin-like growth factor I, and transforming growth factor alpha messenger RNAs and their respective receptor messenger RNAs in primary human gastric carcinomas: in vivo studies with in situ hybridization and immunocytochemistry. Cancer Res. 1992. 52:3453–3459.15. Rosen LS. Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control. 2002. 9:36–44.16. Harmey JH, Bouchier-Hayes D. Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: implications for anti-angiogenic therapy. Bioessays. 2002. 24:280–283.17. Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF, et al. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993. 143:1255–1262.18. Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995. 55:3964–3968.19. Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer. 1997. 79:206–213.20. Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996. 77:858–863.21. Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999. 274:10002–10007.22. Gupta K, Kshirsagar S, Li W, Gui L, Ramakrishnan S, Gupta P, et al. VEGF prevents apoptosis of human microvascular endothelial cells via opposing effects on MAPK/ERK and SAPK/JNK signaling. Exp Cell Res. 1999. 247:495–504.23. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007. 356:125–134.24. Chen CA, Cheng WF, Lee CN, Wei LH, Chu JS, Hsieh FJ, et al. Cytosol vascular endothelial growth factor in endometrial carcinoma: correlation with disease-free survival. Gynecol Oncol. 2001. 80:207–212.25. Liu XH, Yao S, Kirschenbaum A, Levine AC. NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res. 1998. 58:4245–4249.26. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995. 83:493–501.27. Corcoran CA, He Q, Huang Y, Sheikh MS. Cyclooxygenase-2 interacts with p53 and interferes with p53-dependent transcription and apoptosis. Oncogene. 2005. 24:1634–1640.28. Choi EM, Heo JI, Oh JY, Kim YM, Ha KS, Kim JI, et al. COX-2 regulates p53 activity and inhibits DNA damage-induced apoptosis. Biochem Biophys Res Commun. 2005. 328:1107–1112.29. Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000. 105:1589–1594.30. Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. 2002. 190:279–286.31. Gately S. The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 2000. 19:19–27.32. Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M. Cyclooxygenase-2 expression correlates with apoptosis and angiogenesis in endometrial cancer tissue. Anticancer Res. 2007. 27:3765–3770.33. Landen CN Jr, Mathur SP, Richardson MS, Creasman WT. Expression of cyclooxygenase-2 in cervical, endometrial, and ovarian malignancies. Am J Obstet Gynecol. 2003. 188:1174–1176.34. Fujiwaki R, Iida K, Kanasaki H, Ozaki T, Hata K, Miyazaki K. Cyclooxygenase-2 expression in endometrial cancer: correlation with microvessel count and expression of vascular endothelial growth factor and thymidine phosphorylase. Hum Pathol. 2002. 33:213–219.35. Maeda K, Kang SM, Onoda N, Ogawa M, Sawada T, Nakata B, et al. Expression of p53 and vascular endothelial growth factor associated with tumor angiogenesis and prognosis in gastric cancer. Oncology. 1998. 55:594–599.36. Fontanini G, Boldrini L, Vignati S, Chinè S, Basolo F, Silvestri V, et al. Bcl2 and p53 regulate vascular endothelial growth factor (VEGF)-mediated angiogenesis in non-small cell lung carcinoma. Eur J Cancer. 1998. 34:718–723.37. Kieser A, Weich HA, Brandner G, Marmé D, Kolch W. Mutant p53 potentiates protein kinase C induction of vascular endothelial growth factor expression. Oncogene. 1994. 9:963–969.38. Giatromanolaki A, Koukourakis MI, Kakolyris S, Turley H, O'Byrne K, Scott PA, et al. Vascular endothelial growth factor, wild-type p53, and angiogenesis in early operable non-small cell lung cancer. Clin Cancer Res. 1998. 4:3017–3024.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Synergistic Effect of COX-2 Inhibitor on Paclitaxel-Induced Apoptosis in the Human Ovarian Cancer Cell Line OVCAR-3
- In Vitro Response of Uterine Endometrial Cancer Cell Lines to the Antiestrogen Tamoxifen
- Induction of growth inhibition and apoptosis in human endometrial cancer cells by histone deacetylase inhibitors
- Growth inhibition of oral squamous cell carcinorma cell line induced by cox inhibitor
- Knockdown of LKB1 Sensitizes Endometrial Cancer Cells via AMPK Activation