Korean J Obstet Gynecol.
2012 Mar;55(3):143-147. 10.5468/KJOG.2012.55.3.143.
Pharmacotherapy for premenstrual dysphoric disorder: A meta-analysis of phase 3 trials
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea. tkim@kumc.or.kr
- KMID: 2274118
- DOI: http://doi.org/10.5468/KJOG.2012.55.3.143
Abstract
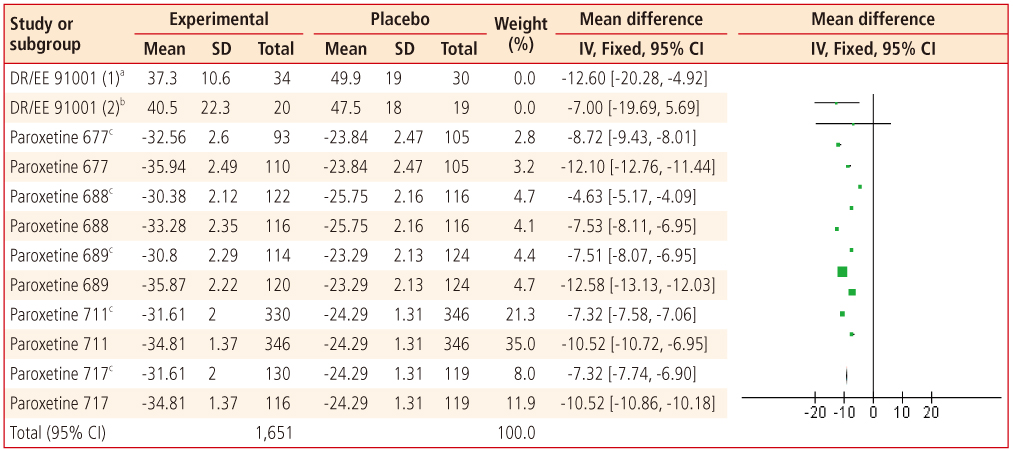
- Premenstrual dysphoric disorder (PMDD) is a common condition that temporarily, but repetitively affects patient's global function. Patients and physicians alike are often uncertain whether prescription medication for PMDD is sufficiently effective. The primary objective of this analysis is signal detection in efficacy of pharmacological treatments in PMDD. Secondary objective is to review which symptoms are likely to respond to which medications. The review included otherwise healthy women with clinician confirmed diagnosis of PMDD who participated in phase 3 clinical trials for the treatment of PMDD. Twelve pair-wise comparisons of drug and placebo for 2,420 patients with PMDD were performed. Oral contraceptives and selective serotonin receptor inhibitor were effective in alleviating symptoms of PMDD compared to placebo. Both Intermittent and continuous administration were more effective than placebo. This meta-analysis provides a signal that pharmacological treatment of PMDD is effective.
MeSH Terms
Figure
Reference
-
1. Logue CM, Moos RH. Perimenstrual symptoms: prevalence and risk factors. Psychosom Med. 1986. 48:388–414.2. Ramcharan S, Love EJ, Fick GH, Goldfien A. The epidemiology of premenstrual symptoms in a population-based sample of 2650 urban women: attributable risk and risk factors. J Clin Epidemiol. 1992. 45:377–392.3. Sveinsdóttir H, Bäckström T, Backstrom T. Menstrual cycle symptom variation in a community sample of women using and not using oral contraceptives. Acta Obstet Gynecol Scand. 2000. 79:757–764.4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR). 2000. 4th ed. Arlington, (VA): American Psychiatric Association.5. Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med. 2002. 32:119–132.6. Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology. 2003. 28:Suppl 3. 1–23.7. Freeman EW. Premenstrual syndrome: current perspectives on treatment and etiology. Curr Opin Obstet Gynecol. 1997. 9:147–153.8. Halbreich U. Premenstrual syndromes: closing the 20th century chapters. Curr Opin Obstet Gynecol. 1999. 11:265–270.9. Mortola JF. Premenstrual syndrome. Trends Endocrinol Metab. 1996. 7:184–189.10. Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005. 72:414–421.11. Clinical trials 91001 [Internet]. Bayer Healthcare. cited 2012 Jan 14. Berlin (DE): Bayer;Available from: http://www.bayerhealthcare.com/scripts/pages/en/research_development/clinical_trials/trial_finder/trialfinder_detail.php?trialid=91001&search=91001&product=&overall_status=&country=&phase=&condition=&results=0&trials=0&btnSubmit=submit&num=30&show=1.12. Clinical study 29060/677 [Internet]. GlaxoSmithKline. 2012. cited 2012 Jan 14. Middlesex (UK): GlaxoSmithKline;Available from: http://www.gsk-clinicalstudyregister.com/result_detail.jsp?protocolId=29060%2f677&studyId=3742A8A5-2C3C-4A40-B9B5-7975DF70CF53&compound=paroxetine.13. Clinical study 29060/688 [Internet]. GlaxoSmithKline. 2012. cited 2012 Jan 14. Middlesex (UK): GlaxoSmithKline;Available from: http://www.gsk-clinicalstudyregister.com/result_detail.jsp;jsessionid=CC6457E95B10471DFE84DE6FF7B160D3?protocolId=29060%2f688&studyId=4EAE38F8-954D-453B-BA8C-32CA4FFAD277&compound=paroxetine.14. Clinical study 29060/689 [Internet]. GlaxoSmithKline. 2012. cited 2012 Jan 14. Middlesex (UK): GlaxoSmithKline;Available from: http://www.gsk-clinicalstudyregister.com/result_detail.jsp;jsessionid=CC6457E95B10471DFE84DE6FF7B160D3?protocolId=29060%2f689&studyId=6F9A5C75-99C3-4F11-AED7-A122263D1746&compound=paroxetine.15. Clinical study 29060/711 [Internet]. GlaxoSmithKline. 2012. cited 2012 Jan 14. Middlesex (UK): GlaxoSmithKline;Available from: http://www.gsk-clinicalstudyregister.com/result_detail.jsp;jsessionid=CC6457E95B10471DFE84DE6FF7B160D3?protocolId=29060%2f711&studyId=4A384A1A-46F7-4E8B-915B-4B324BB64491&compound=paroxetine.16. Clinical study 29060/717 [Internet]. GlaxoSmithKline. 2012. cited 2012 Jan 14. Middlesex (UK): GlaxoSmithKline;Available from: http://www.gsk-clinicalstudyregister.com/result_detail.jsp;jsessionid=CC6457E95B10471DFE84DE6FF7B160D3?protocolId=29060%2f717&studyId=CA9A3538-05F2-43D8-A1E2-342281DDD178&compound=paroxetine.17. PAXIL (paroxetine hydrochloride) tablets and oral suspension [Internet]. GlaxoSmithKline. 2012. cited 2012 Jan 14. Research Triangle Park (NC): GlaxoSmithKline;Available from: http://us.gsk.com/products/assets/us_paxil.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of Premenstrual Dysphoric Disorder and Occupational Function in a Nurse Group
- The Standardization of the Shortened Premenstrual Assessment Form and Applicability on the Internet
- Premenstrual syndrome & premenstrual dysphoric disorder
- Understanding and Treatment of Premenstrual Dysphoric Disorder
- Relationship among Cognitive Style, Perceived Stress and Premenstrual Symptoms