Korean J Obstet Gynecol.
2010 Jul;53(7):565-578. 10.5468/kjog.2010.53.7.565.
A general perspective on breast disease and training system for breast cancer specialists in Europe
- Affiliations
-
- 1Mirae & Heemang Obstetrics and Gynecology Clinic, Seoul, Korea. hoenil@gmail.com
- KMID: 2273937
- DOI: http://doi.org/10.5468/kjog.2010.53.7.565
Abstract
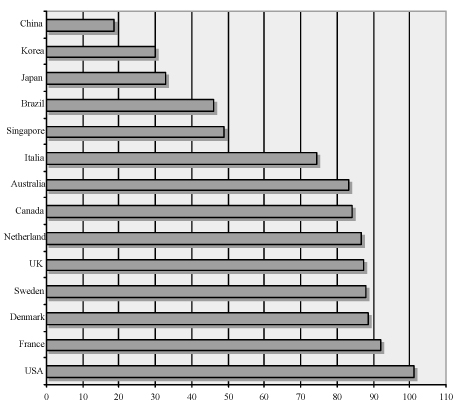
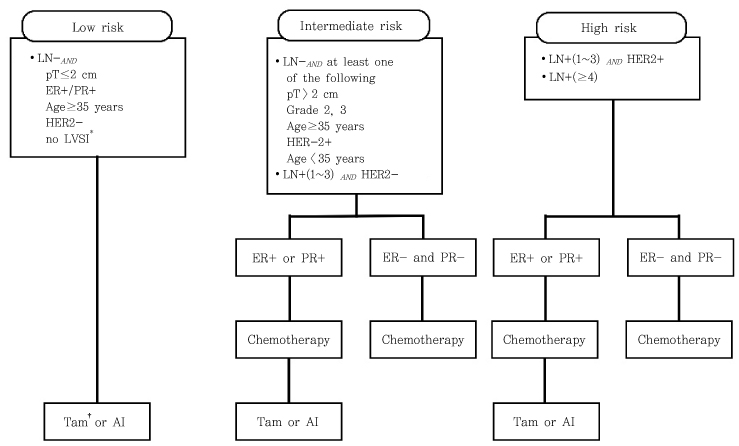
- Breast is a female reproductve organ and breast disease is closely related to female reproductive function. In recent years, becoming the most frequent cancer in female, breast cancer has emerged as a major health concern for Korean women. Obtaining basic knowledge about breast disease is crucial for the specialists dealing with female reproductive organs. In this article a brief overview over the management of breast cancer as well as benign breast disease will be presented. In addition, the current stauts of training system for breast cancer in Europe will be discussed. Examinations of the current practices in Europe will be able to guide the effort in Korea to incorporate breast diseae into the field of gynecology.
Keyword
Figure
Reference
-
1. International Federation of Gynecology and Obstetrics [Internet]. c2010. updated 2010 May 2; cited 2010 Jun 10. London: International Federation of Gynecology and Obstetrics;Availavle from: http://www.figo.org/news/professor-josé-aristodemo-pinotti-1934-2009.2. Vorherr H. Fibrocystic breast disease: pathophysiology, pathomorphology, clinical picture, and management. Am J Obstet Gynecol. 1986. 154:161–179.3. Love SM, Gelman RS, Silen W. Sounding board. Fibrocystic "disease" of the breast-a nondisease? N Engl J Med. 1982. 307:1010–1014.4. Guray M, Sahin AA. Benign breast diseases: classification, diagnosis, and management. Oncologist. 2006. 11:435–449.5. Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985. 312:146–151.6. Dupont WD, Parl FF, Hartmann WH, Brinton LA, Winfield AC, Worrell JA, et al. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 1993. 71:1258–1265.7. Tavassoli FA. Tavassoli FA, editor. Biphasic tumors. Pathology of the breast. 1999. 2nd ed. Stamford, CT: Appleton & Lange;571–623.8. Carter BA, Page DL, Schuyler P, Parl FF, Simpson JF, Jensen RA, et al. No elevation in long-term breast carcinoma risk for women with fibroadenomas that contain atypical hyperplasia. Cancer. 2001. 92:30–36.9. El-Wakeel H, Umpleby HC. Systematic review of fibroadenoma as a risk factor for breast cancer. Breast. 2003. 12:302–307.10. Shabtai M, Saavedra-Malinger P, Shabtai EL, Rosin D, Kuriansky J, Ravid-Megido M, et al. Fibroadenoma of the breast: analysis of associated pathological entities-a different risk marker in different age groups for concurrent breast cancer. Isr Med Assoc J. 2001. 3:813–817.11. Rahal RM, de Freitas-Junior R, Paulinelli RR. Risk factors for duct ectasia. Breast J. 2005. 11:262–265.12. Cancer incidence in five continents. Volume VII. IARC Sci Publ. 1997. (143):1–1240.13. Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. IARC cancer base no. 5 (version 2.0). 2004. Lyon: The International Agency for Research on Cancer (IARC) Press.14. Coleman MP, Esteve J, Damiecki P, Arslan A, Renard H. Trends in cancer incidence and mortality. IARC Sci Publ. 1993. (121):1–806.15. Korean Breast Cancer Society [Internet]. c2009. cited 2010 June 10. Seoul: KBCS;Availavle from: http://www.kbcs.or.kr/.16. Arriagada R, Le MG, Dunant A, Tubiana M, Contesso G. Twenty-five years of follow-up in patients with operable breast carcinoma: correlation between clinicopathologic factors and the risk of death in each 5-year period. Cancer. 2006. 106:743–750.17. Fisher ER, Anderson S, Tan-Chiu E, Fisher B, Eaton L, Wolmark N. Fifteen-year prognostic discriminants for invasive breast carcinoma: National Surgical Adjuvant Breast and Bowel Project Protocol-06. Cancer. 2001. 91:1679–1687.18. Weiss RB, Woolf SH, Demakos E, Holland JF, Berry DA, Falkson G, et al. Natural history of more than 20 years of node-positive primary breast carcinoma treated with cyclophosphamide, methotrexate, and fluorouracil-based adjuvant chemotherapy: a study by the Cancer and Leukemia Group B. J Clin Oncol. 2003. 21:1825–1835.19. Reed W, Hannisdal E, Boehler PJ, Gundersen S, Host H, Marthin J. The prognostic value of p53 and c-erb B-2 immunostaining is overrated for patients with lymph node negative breast carcinoma: a multivariate analysis of prognostic factors in 613 patients with a follow-up of 14-30 years. Cancer. 2000. 88:804–813.20. Schoppmann SF, Bayer G, Aumayr K, Taucher S, Geleff S, Rudas M, et al. Prognostic value of lymphangiogenesis and lymphovascular invasion in invasive breast cancer. Ann Surg. 2004. 240:306–312.21. Frkovic-Grazio S, Bracko M. Long term prognostic value of Nottingham histological grade and its components in early (pT1N0M0) breast carcinoma. J Clin Pathol. 2002. 55:88–92.22. Northridge ME, Rhoads GG, Wartenberg D, Koffman D. The importance of histologic type on breast cancer survival. J Clin Epidemiol. 1997. 50:283–290.23. Vorgias G, Koukouras D, Paleogianni V, Tzoracoeleftherakis E. Prognostic significance of factors affecting disease free interval and overall survival for Stage II breast cancer in Greece. A multivariate cohort study. Eur J Obstet Gynecol Reprod Biol. 2001. 95:100–104.24. Ferrero-Pous M, Hacene K, Bouchet C, Le Doussal V, Tubiana-Hulin M, Spyratos F. Relationship between c-erbB-2 and other tumor characteristics in breast cancer prognosis. Clin Cancer Res. 2000. 6:4745–4754.25. Menard S, Balsari A, Casalini P, Tagliabue E, Campiglio M, Bufalino R, et al. HER-2-positive breast carcinomas as a particular subset with peculiar clinical behaviors. Clin Cancer Res. 2002. 8:520–525.26. Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005. 97:1652–1662.27. Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998. 90:1371–1388.28. Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006. 295:2727–2741.29. Julien JP, Bijker N, Fentiman IS, Peterse JL, Delledonne V, Rouanet P, et al. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. Lancet. 2000. 355:528–533.30. Vargas C, Kestin L, Go N, Krauss D, Chen P, Goldstein N, et al. Factors associated with local recurrence and cause-specific survival in patients with ductal carcinoma in situ of the breast treated with breast-conserving therapy or mastectomy. Int J Radiat Oncol Biol Phys. 2005. 63:1514–1521.31. Morrow M, Strom EA, Bassett LW, Dershaw DD, Fowble B, Giuliano A, et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin. 2002. 52:277–300.32. Solin LJ, Recht A, Fourquet A, Kurtz J, Kuske R, McNeese M, et al. Ten-year results of breast-conserving surgery and definitive irradiation for intraductal carcinoma (ductal carcinoma in situ) of the breast. Cancer. 1991. 68:2337–2344.33. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002. 347:1233–1241.34. Arriagada R, Le MG, Rochard F, Contesso G. Institut Gustave-Roussy Breast Cancer Group. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. J Clin Oncol. 1996. 14:1558–1564.35. Early Breast Cancer Trialists' Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med. 1995. 333:1444–1455.36. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006. 24:2019–2027.37. Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999. 353:1641–1648.38. Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005. 97:116–126.39. Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006. 295:1658–1667.40. Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, Martino S, Perez EA, Muss HB, Norton L, Hudis C, Winer EP. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006. 295:1658–1667.41. Buzdar A, Douma J, Davidson N, Elledge R, Morgan M, Smith R, et al. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J Clin Oncol. 2001. 19:3357–3366.42. Buzdar AU, Jonat W, Howell A, Jones SE, Blomqvist CP, Vogel CL, et al. Arimidex Study Group. Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: results of a survival update based on a combined analysis of data from two mature phase III trials. Cancer. 1998. 83:1142–1152.43. Muss HB, Case LD, Richards F 2nd, White DR, Cooper MR, Cruz JM, et al. The Piedmont Oncology Association. Interrupted versus continuous chemotherapy in patients with metastatic breast cancer. N Engl J Med. 1991. 325:1342–1348.44. Falkson G, Gelman RS, Pandya KJ, Osborne CK, Tormey D, Cummings FJ, et al. Eastern Cooperative Oncology Group randomized trials of observation versus maintenance therapy for patients with metastatic breast cancer in complete remission following induction treatment. J Clin Oncol. 1998. 16:1669–1676.45. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003. 349:546–553.46. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006. 98:599–609.47. Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005. 365:60–62.48. Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1-98. J Clin Oncol. 2007. 25:486–492.49. Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002. 359:2131–2139.50. Piccart MJ, Di Leo A, Beauduin M, Vindevoghel A, Michel J, Focan C, et al. Phase III trial comparing two dose levels of epirubicin combined with cyclophosphamide with cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer. J Clin Oncol. 2001. 19:3103–3110.51. Levine MN, Pritchard KI, Bramwell VH, Shepherd LE, Tu D, Paul N. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol. 2005. 23:5166–5170.52. Bang SM, Heo DS, Lee KH, Byun JH, Chang HM, Noh DY, et al. Adjuvant doxorubicin and cyclophosphamide versus cyclophosphamide, methotrexate, and 5-fluorouracil chemotherapy in premenopausal women with axillary lymph node positive breast carcinoma. Cancer. 2000. 89:2521–2526.53. Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005. 23:3686–3696.54. Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S, et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003. 21:976–983.55. Eniu A, Palmieri FM, Perez EA. Weekly administration of docetaxel and paclitaxel in metastatic or advanced breast cancer. Oncologist. 2005. 10:665–685.56. Conlin AK, Seidman AD. Taxanes in breast cancer: an update. Curr Oncol Rep. 2007. 9:22–30.57. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005. 353:1673–1684.58. Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW Jr, et al. Alcohol, tobacco and breast cancer-collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002. 87:1234–1245.59. Deapen D, Liu L, Perkins C, Bernstein L, Ross RK. Rapidly rising breast cancer incidence rates among Asian-American women. Int J Cancer. 2002. 99:747–750.60. Minami Y, Tsubono Y, Nishino Y, Ohuchi N, Shibuya D, Hisamichi S. The increase of female breast cancer incidence in Japan: emergence of birth cohort effect. Int J Cancer. 2004. 108:901–906.61. Richard MA, Baum M, Dowsett M, et al. Provision of breast services in the UK; the advantage of specialist breast units. Report of a Working Party of the British Breast Group. . 1994. The Breast Supplement.62. Blichert-Toft M, Smola MG, Cataliotti L, O'Higgins N. Principles and guidelines for surgeons--management of symptomatic breast cancer. European Society of Surgical Oncology. Eur J Surg Oncol. 1997. 23:101–109.63. Blamey RW. BASO Breast Specialty Group. The British Association of Surgical Oncology Guidelines for surgeons in the management of symptomatic breast disease in the UK (1998 revision). Eur J Surg Oncol. 1998. 24:464–476.64. Guidance for Purchasers: Improving Outcomes in Breast Cancer-The Manual. 1996. Leeds: NHS Executive Publication.65. The requirements of a specialist breast unit. Eur J Cancer. 2000. 36:2288–2293.66. SenoNetwork [Internet]. 2010. cited 2010 June 25. Milan: SenoNetwork;Availavle from: http://www.senonetwork.org/.67. Merck B, Cansado P, Fernandez-Frias A, Rodriguez-Lescure A, Costa D, Lacueva FJ, et al. Application of the EUSOMA criteria in breast units in countries of the European Union. Cir Esp. 2005. 77:65–69.68. Subspecialist training programme in Gynecological Oncology [Internet]. European Board and College of Obsetricians and Gynaecologists (EBCOG) and European Society of Gynaecological Oncology (ESGO). c2009. cited 2010 June 25. Brussels: Postgraduate training and assessment working Party of EBCOG in cooperation with ESGO;Availavle from: http://www.esgo.org/Education/Documents/1-ESGOEBCOG_Subspecialty_Training_Programme_inGO.pdf.69. Beckmann MW, Gitsch G, Emons G, Berg D, Kaufmann M. Qualification and education in gynecological oncology. Zentralbl Gynakol. 2006. 128:1–4.70. Cibula D, Kesic V. Surgical education and training in gynecologic oncology I: European perspective. Gynecol Oncol. 2009. 114:2 Suppl. S52–S55.71. Cataliotti L, De Wolf C, Holland R, Marotti L, Perry N, Redmond K, et al. Guidelines on the standards for the training of specialised health professionals dealing with breast cancer. Eur J Cancer. 2007. 43:660–675.