Korean J Orthod.
2012 Apr;42(2):64-72. 10.4041/kjod.2012.42.2.64.
Response of masticatory muscles to passive stretch stimulus - from perspectives of functional appliances
- Affiliations
-
- 1Department of Orthodontics and Pediatric Dentistry, University of Maryland, School of Dentistry, Baltimore, MD, USA. epae@umaryland.edu
- KMID: 2273257
- DOI: http://doi.org/10.4041/kjod.2012.42.2.64
Abstract
OBJECTIVE
The aims of this study were to examine whether a passive stretch stimulus by means of a functional appliance induces changes in the fiber composition of masticatory muscles and whether these changes are similar to the changes in stretched limb muscle fibers by using RT-PCR, western blot, and immunohistochemical assays.
METHODS
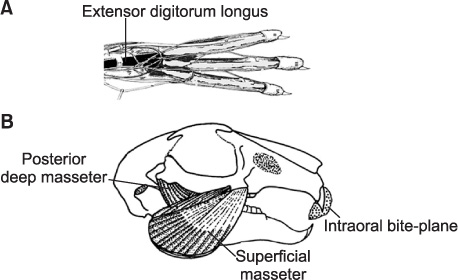
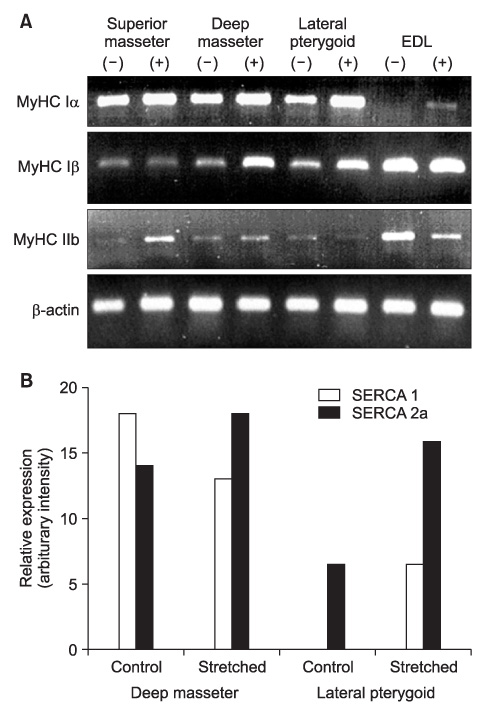
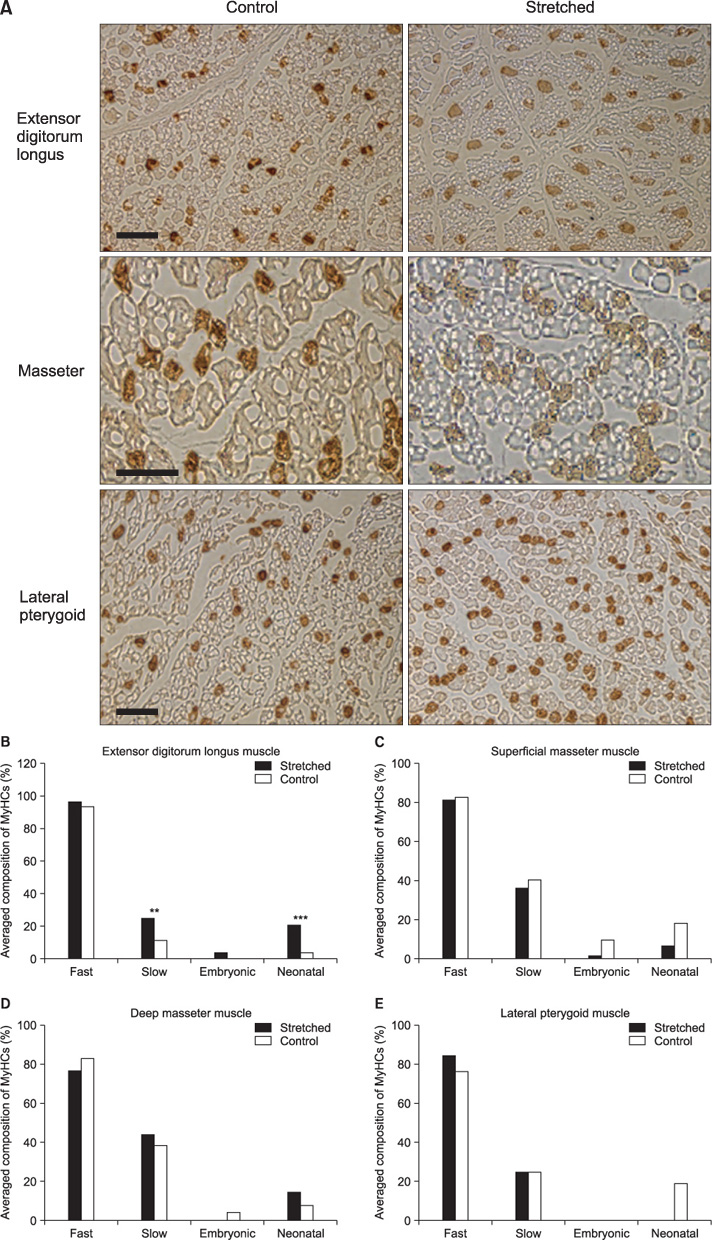
Five male New Zealand White rabbits were fitted with a prefabricated inclined plane on the maxillary central incisors to force the mandible forward (- 2 mm) and downward (- 4 mm). Further, 1 hind limb was extended and constrained with a cast so that the extensor digitorum longus (EDL) was stretched when the animal used the limb. The animals were sacrificed after 1 week and the masseter, lateral pterygoid, and EDL were processed and compared with those from control animals (n = 3).
RESULTS
The stretched EDL had a significantly higher percentage of slow fibers, whereas the stretched masticatory muscles did not show changes in the composition of the major contractile proteins after 7 days.
CONCLUSIONS
The transition of fiber phenotypes in response to a stretch stimulus may take longer in the masticatory muscles than in the limb muscles.
MeSH Terms
Figure
Reference
-
1. Goldspink G, Scutt A, Loughna PT, Wells DJ, Jaenicke T, Gerlach GF. Gene expression in skeletal muscle in response to stretch and force generation. Am J Physiol. 1992. 262:R356–R363.
Article2. Goldspink G. Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat. 1999. 194:323–334.
Article3. Pattullo MC, Cotter MA, Cameron NE, Barry JA. Effects of lengthened immobilization on functional and histochemical properties of rabbit tibialis anterior muscle. Exp Physiol. 1992. 77:433–442.
Article4. Williams P, Watt P, Bicik V, Goldspink G. Effect of stretch combined with electrical stimulation on the type of sarcomeres produced at the ends of muscle fibers. Exp Neurol. 1986. 93:500–509.
Article5. Yang H, Alnaqeeb M, Simpson H, Goldspink G. Changes in muscle fibre type, muscle mass and IGF-I gene expression in rabbit skeletal muscle subjected to stretch. J Anat. 1997. 190:613–622.
Article6. Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990. 116:1–76.
Article7. Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000. 50:500–509.
Article8. Essig DA, Devol DL, Bechtel PJ, Trannel TJ. Expression of embryonic myosin heavy chain mRNA in stretched adult chicken skeletal muscle. Am J Physiol. 1991. 260:C1325–C1331.
Article9. Ohnuki Y, Saeki Y, Yamane A, Kawasaki K, Yanagisawa K. Adaptation of guinea-pig superficial masseter muscle to an increase in occlusal vertical dimension. Arch Oral Biol. 1999. 44:329–335.
Article10. Gedrange T, Luck O, Hesske G, Büttner C, Seibel P, Harzer W. Differential expression of myosin heavy-chain mRNA in muscles of mastication during functional advancement of the mandible in pigs. Arch Oral Biol. 2001. 46:215–220.
Article11. Easton JW, Carlson DS. Adaptation of the lateral pterygoid and superficial masseter muscles to mandibular protrusion in the rat. Am J Orthod Dentofacial Orthop. 1990. 97:149–158.
Article12. Sfondrini G, Reggiani C, Gandini P, Bovenzi R, Pellegrino MA. Adaptations of masticatory muscles to a hyperpropulsive appliance in the rat. Am J Orthod Dentofacial Orthop. 1996. 110:612–617.
Article13. Petrovic A, Stutzmann J, Oudet C. McNamara JA, Carlson DS, Ribbens KA, editors. Defects in mandibular growth resulting from condylectomy and resection of the pterygoid and masseter muscles. The effect of surgical intervention on craniofacial growth, Mono graph 12, Craniofacial growth series. 1982. Ann Arbor: Center for Human Growth and Development.14. Loughna PT, Izumo S, Goldspink G, Nadal-Ginard B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development. 1990. 109:217–223.
Article15. Loughna P, Goldspink G, Goldspink DF. Effect of inactivity and passive stretch on protein turnover in phasic and postural rat muscles. J Appl Physiol. 1986. 61:173–179.
Article16. Pae EK, Hyatt JP, Wu J, Chien P. Short-term electrical stimulation alters tongue muscle fibre type composition. Arch Oral Biol. 2007. 52:544–551.
Article17. Jaschinski F, Schuler M, Peuker H, Pette D. Changes in myosin heavy chain mRNA and protein isoforms of rat muscle during forced contractile activity. Am J Physiol. 1998. 274:C365–C370.18. Eizema K, van der Wal DE, van den Burg MM, de Jonge HW, Everts ME. Differential expression of calcineurin and SR Ca2+ handling proteins in equine muscle fibers during early postnatal growth. J Histochem Cytochem. 2007. 55:247–254.
Article19. Hori A, Ishihara A, Kobayashi S, Ibata Y. Immunohistochemical classification of skeletal muscle fibers. Acta Histochem Cytochem. 1998. 31:375–384.
Article20. Bredman JJ, Weijs WA, Korfage HA, Brugman P, Moorman AF. Myosin heavy chain expression in rabbit masseter muscle during postnatal development. J Anat. 1992. 180:263–274.21. Jacobs-El J, Zhou MY, Russell B. MRF4, Myf-5, and myogenin mRNAs in the adaptive responses of mature rat muscle. Am J Physiol. 1995. 268:C1045–C1052.
Article22. Rathbone CR, Wenke JC, Warren GL, Armstrong RB. Importance of satellite cells in the strength recovery after eccentric contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2003. 285:R1490–R1495.
Article23. Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol. 2003. 549:409–418.
Article24. Chen HL, Nosaka K, Chen TC. Muscle damage protection by low-intensity eccentric contractions remains for 2 weeks but not 3 weeks. Eur J Appl Physiol. 2012. 112:555–565.
Article25. Hägg U, Rabie AB, Bendeus M, Wong RW, Wey MC, Du X, et al. Condylar growth and mandibular positioning with stepwise vs maximum advancement. Am J Orthod Dentofacial Orthop. 2008. 134:525–536.
Article26. Bredman JJ, Wessels A, Weijs WA, Korfage JA, Soffers CA, Moorman AF. Demonstration of 'cardiac-specific' myosin heavy chain in masticatory muscles of human and rabbit. Histochem J. 1991. 23:160–170.
Article27. Bredman JJ, Weijs WA, Moorman AF, Brugman P. Histochemical and functional fibre typing of the rabbit masseter muscle. J Anat. 1990. 168:31–47.28. Rowlerson A, Pope B, Murray J, Whalen RB, Weeds AG. A novel myosin present in cat jaw-closing muscles. J Muscle Res Cell Motil. 2000. 2:415–438.
Article29. Sciote JJ, Morris TJ. Skeletal muscle function and fibre types: the relationship between occlusal function and the phenotype of jaw-closing muscles in human. J Orthod. 2000. 27:15–30.
Article30. Horton MJ, Brandon CA, Morris TJ, Braun TW, Yaw KM, Sciote JJ. Abundant expression of myosin heavy-chain IIB RNA in a subset of human masseter muscle fibres. Arch Oral Biol. 2001. 46:1039–1050.
Article31. Widmer CG, Morris-Wiman JA, Nekula C. Spatial distribution of myosin heavy-chain isoforms in mouse masseter. J Dent Res. 2002. 81:33–38.
Article32. Ohnuki Y, Saeki Y, Yamane A, Yanagisawa K. Quantitative changes in the mRNA for contractile proteins and metabolic enzymes in masseter muscle of bite-opened rats. Arch Oral Biol. 2000. 45:1025–1032.
Article33. Ohnuki Y, Kawai N, Tanaka E, Langenbach GE, Tanne K, Saeki Y. Effects of increased occlusal vertical dimension on daily activity and myosin heavy chain composition in rat jaw muscle. Arch Oral Biol. 2009. 54:783–789.
Article34. Korfage JA, Koolstra JH, Langenbach GE, van Eijden TM. Fiber-type composition of the human jaw muscles--(part 2) role of hybrid fibers and factors responsible for inter-individual variation. J Dent Res. 2005. 84:784–793.
Article35. Korfage JA, Koolstra JH, Langenbach GE, van Eijden TM. Fiber-type composition of the human jaw muscles--(part 1) origin and functional significance of fiber-type diversity. J Dent Res. 2005. 84:774–783.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of Intermaxillary Traction Appliances and Exercises to Strengthen the Masticatory Muscles of Patients with Anterior Open Bite Caused by Temporomandibular Joint Osteoarthritis: Case Reports
- The Effect of Passive Stretching on the Spasticity of Ankle Plantar Flexor Muscles
- Trismus casued by inverse activity of masticatory muscles
- Functional Anatomy of the Temporomandibular Joint and Pathologic Changes in Temporomandibular Disease Progression: A Narrative Review
- Myositis involving masticatory muscles in behcet's disease