Blood Res.
2013 Sep;48(3):185-192. 10.5045/br.2013.48.3.185.
Evaluation of prognostic factors in patients with therapy-related acute myeloid leukemia
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. hschi@amc.seoul.kr
- KMID: 2270704
- DOI: http://doi.org/10.5045/br.2013.48.3.185
Abstract
- BACKGROUND
Therapy-related AML (t-AML) occurs as a late complication of chemotherapy administered to treat a prior disorder. Prognostic factors affecting the clinical outcome in t-AML have not yet been clearly defined; therefore, we evaluated these factors in this study.
METHODS
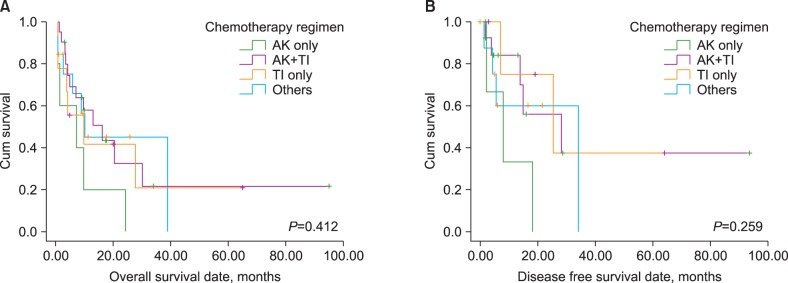
Forty-eight patients diagnosed with t-AML within the past 10 years were enrolled, and their chemotherapy regimens categorized into 4 groups: alkylating agents (AK) only, topoisomerase II inhibitors (TI) and AK, TI only, and others. The prognostic factors affecting clinical outcome were evaluated.
RESULTS
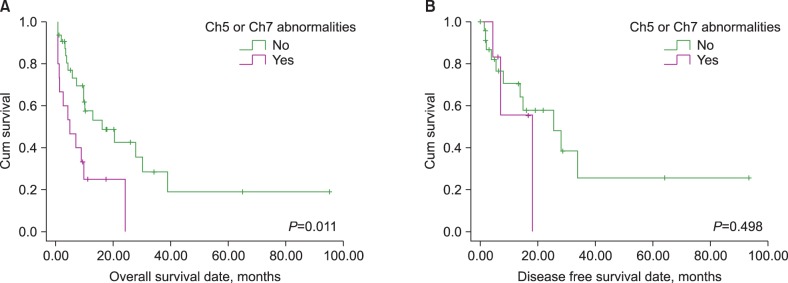
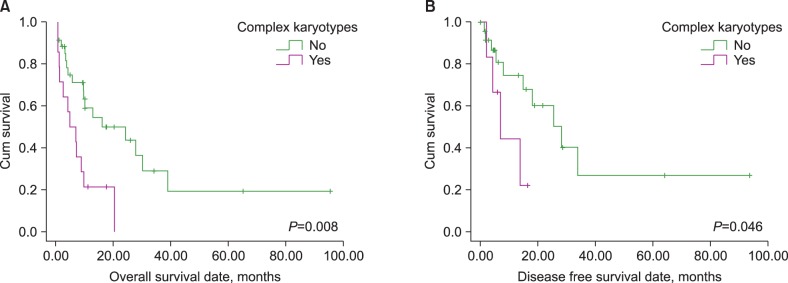
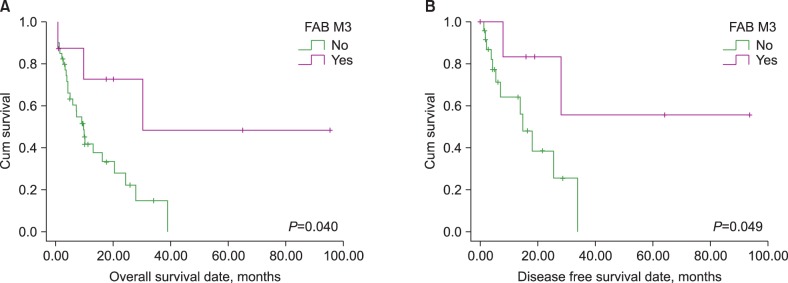
Five (10.4%), 21 (43.8%), 9 (18.8%), and 13 (27.0%) patients were treated with AK only, AK and TI, TI only, and others, respectively. Patients with an AML M3 phenotype showed significantly longer overall survival (OS; 55.1 vs. 14.3 months, P=0.040) and disease-free survival (DFS; 61.2 vs. 17.5 months, P=0.049) than other phenotypes. In contrast, patients with a complex karyotype showed significantly shorter OS (7.9 vs. 31.3 months, P=0.008) and DFS (9.5 vs. 38.6 months, P=0.046); additionally, patients with chromosome 5 or 7 abnormalities showed significantly shorter OS (9.1 vs. 30.7 months, P=0.011) than other phenotypes. Only the presence of a complex karyotype or AML M3 phenotype retained prognostic impact in a multivariate analysis.
CONCLUSION
Only the AML M3 phenotype was identified as having a good prognosis, and this might suggest that it exhibits unique clinical features in t-AML patients. Moreover, our findings indicated that karyotype was the strongest prognostic indicator and predicted a poor prognosis for t-AML patients with a complex karyotype.
MeSH Terms
Figure
Cited by 2 articles
-
Characteristics of hematologic malignancies with coexisting t(9;22) and inv(16) chromosomal abnormalities
Eunhee Han, Hyeyoung Lee, Myungshin Kim, Yonggoo Kim, Kyungja Han, Sung-Eun Lee, Hee-Je Kim, Dong-Wook Kim
Blood Res. 2014;49(1):22-28. doi: 10.5045/br.2014.49.1.22.Therapy-related myeloid neoplasms after transcatheter arterial chemoembolization for hepatocellular carcinoma
Jun Ho Yi, Jun Ho Jang, Chul Won Jung
Blood Res. 2021;56(4):349-353. doi: 10.5045/br.2021.2021197.
Reference
-
1. Pedersen-Bjergaard J, Andersen MK, Johansson B. Balanced chromosome aberrations in leukemias following chemotherapy with DNA-topoisomerase II inhibitors. J Clin Oncol. 1998; 16:1897–1898. PMID: 9586907.
Article2. Cortes J, OBrien S, Kantarjian H, et al. Abnormalities in the long arm of chromosome 11 (11q) in patients with de novo and secondary acute myelogenous leukemias and myelodysplastic syndromes. Leukemia. 1994; 8:2174–2178. PMID: 7808007.3. Quesnel B, Kantarjian H, Bjergaard JP, et al. Therapy-related acute myeloid leukemia with t(8;21), inv(16), and t(8;16): a report on 25 cases and review of the literature. J Clin Oncol. 1993; 11:2370–2379. PMID: 8246025.
Article4. Leone G, Mele L, Pulsoni A, Equitani F, Pagano L. The incidence of secondary leukemias. Haematologica. 1999; 84:937–945. PMID: 10509043.5. Pui CH, Relling MV, Rivera GK, et al. Epipodophyllotoxin-related acute myeloid leukemia: a study of 35 cases. Leukemia. 1995; 9:1990–1996. PMID: 8609707.6. Michels SD, McKenna RW, Arthur DC, Brunning RD. Therapy-related acute myeloid leukemia and myelodysplastic syndrome: a clinical and morphologic study of 65 cases. Blood. 1985; 65:1364–1372. PMID: 3857944.
Article7. Singh ZN, Huo D, Anastasi J, et al. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 2007; 127:197–205. PMID: 17210514.8. Grossmann V, Schnittger S, Kohlmann A, et al. A novel hierarchical prognostic model of AML solely based on molecular mutations. Blood. 2012; 120:2963–2972. PMID: 22915647.
Article9. Kuhnl A, Grimwade D. Molecular markers in acute myeloid leukaemia. Int J Hematol. 2012; 96:153–163. PMID: 22791509.
Article10. Kayser S, Dohner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011; 117:2137–2145. PMID: 21127174.
Article11. Antonijevic N, Suvajdzic N, Terzic T, et al. Favourable prognostic factors in therapy related acute myeloid leukaemia. Srp Arh Celok Lek. 2011; 139:347–352. PMID: 21858974.
Article12. Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapyrelated acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004; 18:120–125. PMID: 14586477.
Article13. Huh HJ, Lee SH, Yoo KH, et al. Therapy-related myeloid neoplasms in 39 Korean patients: a single institution experience. Ann Lab Med. 2013; 33:97–104. PMID: 23483787.
Article14. Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003; 102:43–52. PMID: 12623843.
Article15. Shim H, Chi HS, Jang S, et al. Therapy-related acute leukemia in breast cancer patients: twelve cases treated with a topoisomerase inhibitor. Korean J Hematol. 2010; 45:177–182. PMID: 21120206.
Article16. Andersen MK, Larson RA, Mauritzson N, Schnittger S, Jhanwar SC, Pedersen-Bjergaard J. Balanced chromosome abnormalities inv(16) and t(15;17) in therapy-related myelodysplastic syndromes and acute leukemia: report from an international workshop. Genes Chromosomes Cancer. 2002; 33:395–400. PMID: 11921273.
Article17. Detourmignies L, Castaigne S, Stoppa AM, et al. Therapy-related acute promyelocytic leukemia: a report on 16 cases. J Clin Oncol. 1992; 10:1430–1435. PMID: 1517786.
Article18. Beaumont M, Sanz M, Carli PM, et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003; 21:2123–2137. PMID: 12775738.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent advances in the treatment of pediatric acute leukemia
- Precursor B-Cell Acute Lymphoblastic Leukemia in Two Patients with a History of Cytotoxic Therapy
- Treatments for children and adolescents with AML
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy
- Therapy-related Acute Myeloid Leukemia Following Treatment for Burkitt's Lymphoma